Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2020)Volume 10, Issue 1
Background: Anti-inflammatory and analgesic properties are displayed by several natural products, in particular Acmella oleracea and Zingiber officinale. Literature data suggests that both botanical extracts can be useful as inflammation/pain modulators, so technological efforts have been made to associate them together in a unique delivery new food-grade formulation, Mitidol, with the purpose of improving their beneficial effects.
Objective: The aim of the present work was to assess if Mitidol and its active ingredients are able to modulate the endocannabinoid 2 receptor system, one of the major systems responsible for positive effects of Cannabis treatment. Cannabinoid 2 receptor represents an attractive pharmacological target in obtaining an anti-inflammatory/analgesic effect with low central nervous system side-effects.
Methods: The new food-grade Phytosome, together with all the botanical ingredients and active compounds, were tested in a cell-based assay in human recombinant Cannabinoid 2 Receptor cells, in order to evaluate a possible agonist effect on those receptors, and in a Fatty Acid Amide Hydrolase Inhibition Activity assay, to evaluate potential inhibition of that hydrolase, which is responsible for degradation of the endogenous cannabinoid anandamide.
Results: Zingiber officinale showed potent activity in Fatty Acid Amide Hydrolase Inhibition Activity assay, being about six fold more effective than the flavonoid Kaempferol. Acmella oleracea proved to be highly active in Fatty Acid Amide Hydrolase inhibition and Cannabinoid 2 activation.
Conclusion: Our data suggest a strong rationale for the use of Mitidol as a natural adjuvant in pain management.
Mitidol; Phytosome; FAAH; CB2 receptors; Pain; Inflammation; Acmella oleracea; Zingiber officinale
Plant extracts were extensively used in medicine for several centuries to treat a variety of human discomforts, including pain and inflammation [1]. Both Zingiber officinale [2] and Acmella oleracea [3] are botanical extracts of well-known anti-inflammatory and analgesic actions, involving several pathways, enzymes and receptors.
Gingerols and Shogaols, the major active principles of Zingiber officinale, inhibit crucial enzymes related to inflammation processes, including cyclo-oxygenase COX-2, prostaglandin synthetase, nitric oxide synthase iNOS and 5-lipoxygenase [4-6]. Gingerols are also potent agonists to the vanilloid receptor (TRPV1), a receptor involved in nociceptive pathways [7]. A significant reduction of allodynia and thermal hyperalgesia after intrathecal administration of [6]-gingerol in a peripheral neuropathic pain model in rats was described [8].
Regarding Acmella oleracea extract, the phytochemical profile is characterized by alkylamides, mainly represented by Spilanthol [9]. Acmella extract displayed down-regulating effects of lipopolysaccharide-induced inflammatory mediators, in particular COX-2, iNOS and proinflammatory cytokines, in an in vitro murine macrophage model [10]. Furthermore, antiallodynic and anti-oedematogenic activities in a carrageenan mouse model of an alkylamide-enriched fraction from Acmella oleracea were very recently described [11].
Mitidol® is a rational combination of Zingiber officinale and Acmella oleracea CO2 extracts, formulated using the Phytosome® technology [12]. This formulative technique, successfully applied to deliver natural active compounds like quercetin [13] and bergamot polyphenolic fraction [14], allowed improved oral solubility and consequent oral absorption in humans of botanicals by using a food-grade lecithin formulation.
The present study aims to investigate for the first time a possible interaction of Mitidol and its main components with the endocannabinoid pathway, a highly involved system in both pain and inflammatory modulation [15-18] by using two in vitro assays. The first was a cell-based assay for the human cannabinoid 2 (CB2) receptor in a Chinese hamster ovary cell line, the CHO-K1 cells, to test a possible direct agonist effect on CB2 receptors. In addition, the Fatty Acid Amide Hydrolase (FAAH) Inhibition Activity assay was performed to evaluate a potential inhibition of FAAH, i.e., the main enzyme responsible for the rapid catabolism of Anandamide (AEA), the endogenous ligand that binds and activates both CB1 (Ki< 50 nM) and CB2 receptors (Ki<250 nM) [19]. FAAH represents a key enzyme in the cannabinoid system, due to its ability to hydrolyze anandamide; FAAH inhibition allows blocking of anandamide degradation, resulting in a prolongation and extension of the analgesic effect of anandamide itself [20-22]. The possibility of addressing both CB2 and FAAH targets with one single product of botanical origin could be an original and safe solution for the modulation of inflammatory pain, especially in NSAIDs intolerant subjects, a common syndrome that impairs the use of these anti-inflammatory drugs [23].
The last discovered endogenous cannabinoid Palmitoylethanolamide (PEA), together with Piper nigrum and Myrrh dry natural extracts that showed analgesic/anti-inflammatory activities in classical in vivo tests in rodents i.e., tail immersion, hot plates, writhing test [24,25], were tested in the present study for comparative purposes.
Materials
Zingiber officinale and Acmella oleracea extracts were prepared by carbon dioxide under supercritical conditions. Both extracts are standardized to contain according to HPLC assay: 25% Gingerols and 3% Shogaols for Zingiber, 25% Alkylamides for Acmella extract. Analytical Standards 6, 8 and 10-Gingerols and 6-Shogaol were purchased from Sigma-Aldrich Srl (Italy), Spilanthol from Santa Cruz Biotechnologies, Dodeca-2E, 4E, 8Z, 10E, Z-tetraenoic acid isobutylamide (DTI) from PhytoLab (Germany), Palmitoylethanolamide (PEA) from Fraupharma (Agrate Brianza, Monza Brianza, Italy). Cannabidiol (CBD) was from Indena (Milan, Italy) collection of Analytical Reference Standard samples. Piper nigrum extract (Black pepper 95% Piperine) was purchased from Nutraceutica Srl (Monterenzio, Bologna, Italy) and Myrrh dry extract (Commiphora myrrha) from Solimè Srl (Cavriago, Reggio Emilia, Italy). The combination of Zingiber officinale and Acmella oleracea CO2 extracts was formulated by Indena S.p.A. as Phytosome (Mitidol) [12], a sunflower lecithin delivery system. In the combination, Mitidol consisted of the two standardized extracts in a 5:1 ratio; they were additionally combined with sunflower lecithin in a 1:1 weight ratio (expressed as sum of the botanical extracts and lecithin, respectively). Food additives were added as previously described by Rondanelli et al. in order to improve the physical properties of the Phytosome. All the reagents for cell culture were acquired by Euroclone, except for Ultraglutamine (BioWhitaker) and G418 (InvivoGen). The Fatty Acid Amide Hydrolase Inhibitor Screening Assay Kit was acquired from Cayman Chemical (USA), the CB2 agonist HU210 from Tocris. The substrate of calcium photoprotein coelenterazine was purchased from Biosynth AG; the remaining reagents were supplied by Sigma-Aldrich Srl (Italy).
Fatty Acid Amide Hydrolase (FAAH) inhibition assay
According to the manufacturer’s Protocol, FAAH inhibition was assayed in 96-well fluorescence plates (black). Briefly, 10 μl of each test item was added to 170 μl of assay buffer (125 mM TRIS-HCl, pH 9.0, containing 1 mM EDTA) and 10 μl of FAAH solution (human recombinant enzyme). A control sample with 100% activity was prepared by adding to the well 10 μl of pure DMSO (instead of the test item), 170 μl buffer and 10 μl of FAAH solution, together with a blank sample containing 180 μl of assay buffer and 10 μl of pure DMSO, without FAAH solution. All the samples, in triplicate, were incubated at 37°C for 5 min, then 10 μl of FAAH Substrate Solution (400 μM Arachidonoyl amide) were added to start the enzymatic reaction. The plate was sealed and incubated for 30 min at 37°C and then read using an excitation wavelength of 340-360 nm and an emission wavelength of 450-465 nm. The average fluorescence of the blank samples was subtracted from that of the 100% activity and inhibitor wells. The difference between 100% average activity and each inhibitor sample value was calculated and divided by 100% average activity. The average of these ratios, multiplied by 100, gave the percent inhibition for each product.
A solution of JZL 195 ((4-Nitrophenyl) 4-[(3-phenoxyphenyl) methyl] piperazine-1-carboxylate), a known FAAH inhibitor, was supplied with the kit and used as positive control. The FAAH inhibitors Cannabidiol and Kaempferol were tested in the same assay.
For the most active products, IC50 (concentration at which there was 50% inhibition) was determined, from a graph of Percent Inhibition vs Inhibitor Concentration, by GraphPad software.
Human cannabinoid receptors (hCB2) cell-based assay
The direct agonist activity on human CB2 receptor was evaluated on an established cell-based assay for the human CB2 (CHOchAMPion- hCB2) using EMCCD camera FLIPR Tetra (MDC) technology.
Cell lines: CHO-chAMPion cell line stably co-expressing a calcium activated photoprotein, a cyclic nucleotide-gated (CNG) channel acting as cAMP biosensor and the human CB2 receptor, together with the corresponding CHO-champion Mock cell lines not expressing CB2 receptor were grown in Dulbecco’s MEM/Nutrient Mix F12 (1:1) (DMEM-F12), 1.35 mM Sodium Pyruvate, 11 mM Hepes, 0.2% Sodium Bicarbonate, 10% FBS (Fetal Bovine Serum), 1% Penicillin/Streptomycin, 2 mm Ultraglutamine (200 mM in 0.85% NaCl solution), 1 mg/ml G418 Sulfate.
Mock cell line was used as negative control to determine any nonspecific effects.
Cell-based assay: Sterile assay buffers utilized were Standard Tyrode’s (130 mM NaCl, 5 mM KCl, 2 mM CaCl2, 5 mM NaHCO3, 1 mM MgCl2, 20 mM HEPES, pH 7.4) and calcium free Tyrode’s (130 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM NaHCO3, 20 mM HEPES, pH 7.4).
Dilution of samples was performed by the automated simultaneous pipetting platform CyBio® Well vario (Analytik Jena AG, Germany) as follows: starting from 10 mg/ml (Zingiber officinale CO2, Acmella oleracea CO2 extracts) and 10 mM (6-Gingerol, Spilanthol) stock solutions, serial dilutions (eight concentrations) were performed in 100% DMSO, and further diluted in calcium free Tyrode’s buffer. The final test item concentrations (full dose response curve in quadruplicate) were: 50, 15.8, 5, 1.58, 0.5, 0.15, 0.05 and 0.015 μM for compounds or μg/ml for extracts, with a final DMSO amount of 0.5%.
The assay was performed in 384-well format according to the following protocol: 10,000 cells/well were seeded in 384 multiwell plate in cell culture medium. 24 hours after seeding, the culture medium was removed and cells were loaded with 25 μL/well of coelenterazine (10 μM final concentration) in calcium free Tyrode’s buffer. After 4 hours of incubation at 37°C the well volume was partially removed by aspiration in order to reach 20 μL/well. 10 μl of test items and controls (4X concentrated) in calcium free Tyrode’s buffer were added to the wells. The adenylyl cyclase direct activator Forskolin 10 μl/well (10 μM final concentration) was then added. After 15 min of incubation at room temperature 15 μL/well of 10 mM calcium Tyrode’s buffer (3.3 mM calcium final concentration) were added by the FLIPRTETRA and the photoprotein luminescence signal given by calcium influx was monitored by the instrument over a period of 180 seconds.
The CB2 agonist HU210 at EC100 (1 μM) was used as positive control (100% activity), while buffer represented the negative control (0% activity).
For data quality and data analysis, Genedata Screener® 14.0.5 was used. Compound EC50 was evaluated on percent activity data. The percent activity data were obtained normalizing response values versus agonist at EC100 (Positive control-100% activity) and buffer (Negative control-0% activity).
Effect on FAAH inhibition assay
In order to evaluate the potential of Mitidol to modulate CB1 and CB2 receptors, the FAAH inhibition assay was also performed with single extracts and active principles. Chemical structures of Zingiber officinale and Acmella oleracea active principles are reported in Figure 1.
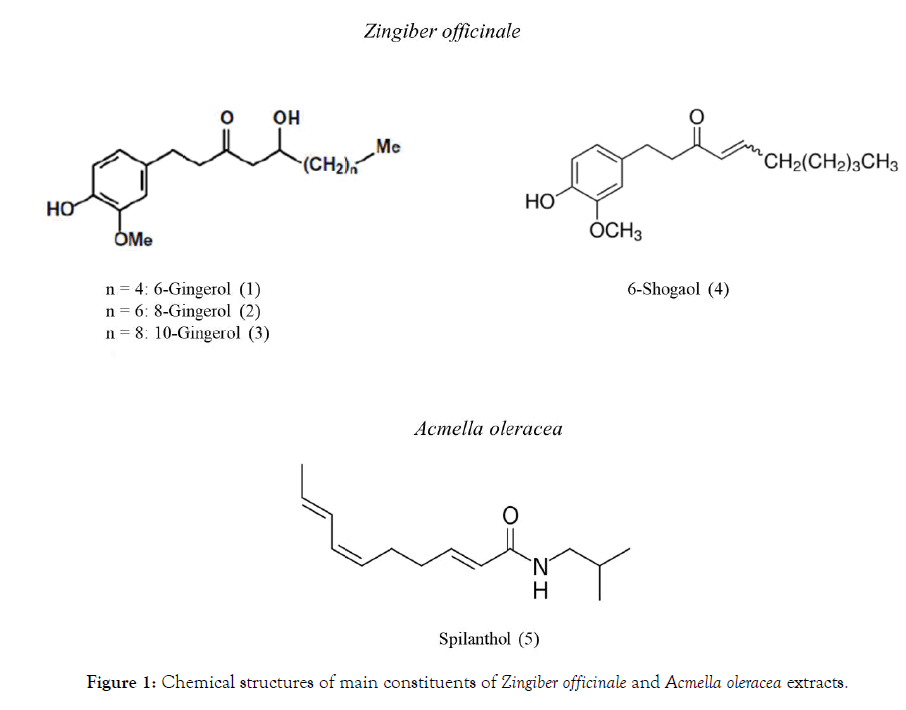
Figure 1: Chemical structures of main constituents of Zingiber officinale and Acmella oleracea extracts.
In FAAH-inhibition assay, all the extracts and active principles were first tested (in triplicate) at the fixed concentration of 200 ppm (except for Spilanthol, 110 ppm), and percentage inhibition of enzyme activity observed was calculated and expressed as Mean ± S.D. Results obtained in enzyme assays are shown in Table 1 and Figure 2 (FAAH Inhibition at fixed concentration). JZL- 195, an active and potent FAAH inhibitor, was also tested in the same experiments, as the positive control for the accuracy of the experimental test. Almost all the samples caused greater than 50% inhibition at the tested concentration, and in particular Acmella oleracea (86%) and Zingiber officinale together with 6-Shogaol producing a complete (100%) inhibition. In terms of active principles of Zingiber officinale the following order of potency was observed: 6-Shogaol>>8-Gingerol>6-Gingerol≅10-Gingerol >>Spilanthol. Mitidol at 200 ppm concentration showed 48.00 ± 2.11% inhibition as expected, keeping in mind that it contains only 10-12% of active principles.
| Sample | Concentration (Ppm) | % Inhibition Mean ± S.D. |
|---|---|---|
| Natural extracts | ||
| Acmella oleracea | 200 | 86.14 ± 0.87 |
| Zingiber officinalis | 200 | 105.06 ± 0.12 |
| Mitidol | 200 | 48.00 ± 2.11 |
| Active principles | ||
| 6-Gingerol (1) | 200 | 67.99 ± 2.03 |
| 8-Gingerol (2) | 200 | 79.98 ± 1.60 |
| 10-Gingerol (3) | 200 | 60.79 ± 3.08 |
| 6-Shogaol (4) | 200 | 102.87 ± 0.37 |
| Spilanthol (5) | 110 | 26.58 ± 5.40 |
| Standards | ||
| Kaempferol | 200 | 102.71 ± 0.62 |
| Cannabidiol | 200 | 59.12 ± 5.79 |
| JZL-195 (positive control) | 0.433 (=1 μM) | 100.24 ± 0.38 |
Table 1: Effect on FAAH inhibition assay at a fixed concentration.
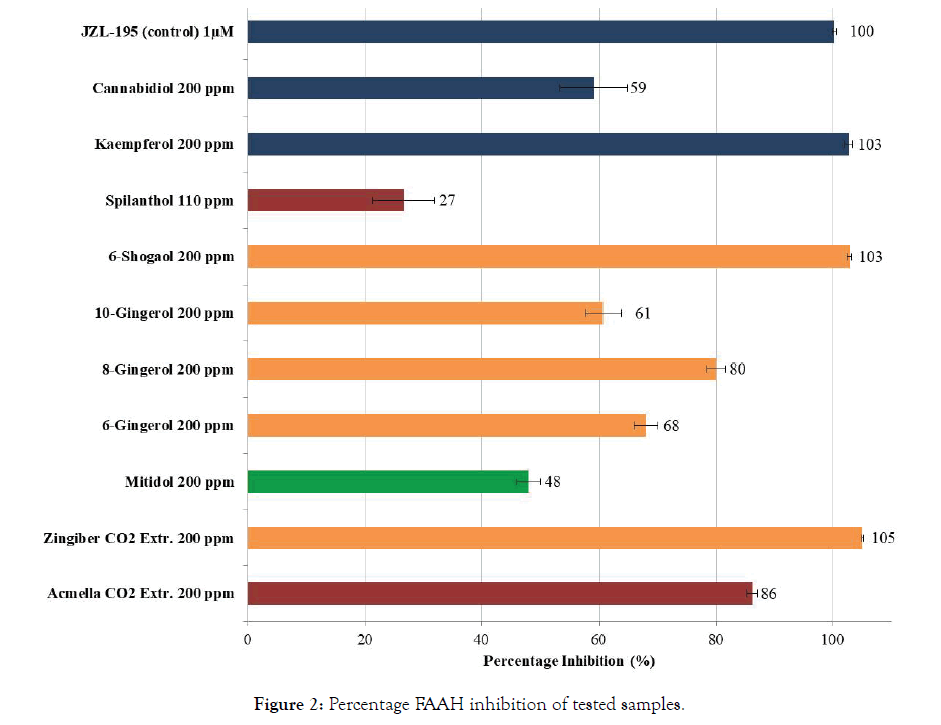
Figure 2: Percentage FAAH inhibition of tested samples.
For the most potent samples, a concentration response curve was performed to obtain IC50 values, as shown in Table 2 and Figure 3, together with those of JZL-195 (positive control) and Kaempferol. Both Acmella oleracea and 6-Shogaol showed a similar IC50, while Zingiber was 2-3 times more potent.
| Sample | IC50 (95% CI) |
|---|---|
| Natural extracts | Ppm |
| Acmella oleracea | 28.55 (19.68-40.09) |
| Zingiber officinalis | 10.33 (7.08-12.72) |
| Active principle | μM |
| 6-Shogaol | 21.2 (19.0-24.4) |
| Standards | μM |
| Kaempferol | 60.99 (37.36-85.74) |
| JZL-195 (positive control) |
0.044 (0.020-0.093) |
Table 2: Inhibitory potency (IC50) on FAAH inhibition assay.
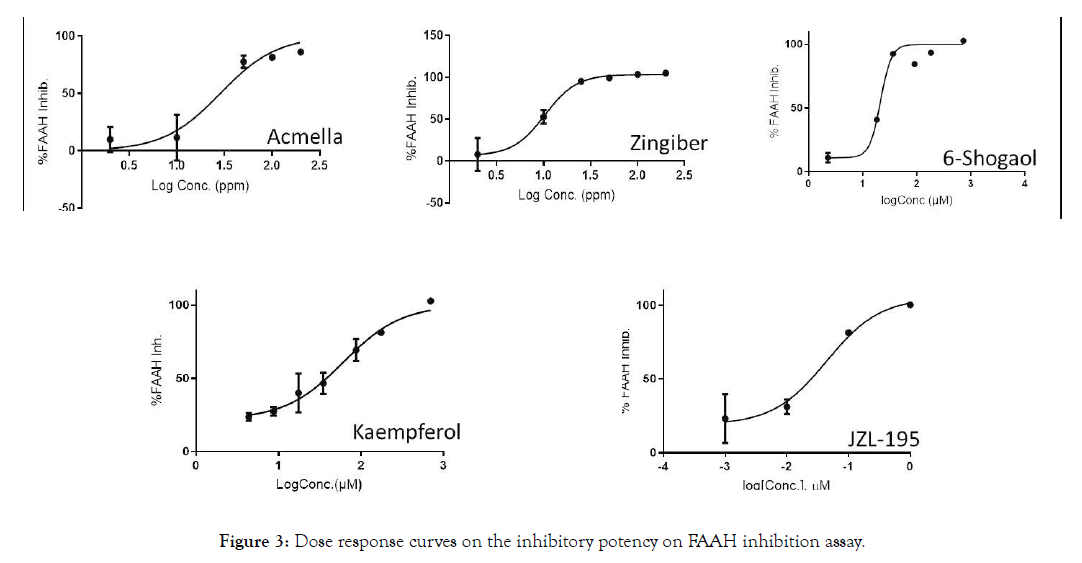
Figure 3: Dose response curves on the inhibitory potency on FAAH inhibition assay.
JZL-195 was active as expected, and Kaempferol activity was in agreement with results obtained in other FAAH inhibition assay models [26].
Zingiber officinale resulted as the most effective tested sample, being about six fold more active than Kaempferol, known from the literature as the most potent FAAH inhibitor among the commonly occurring flavonoids [26].
Effect on human CB2 cell-based assay
In order to assess a possible direct agonist effect of Mitidol on CB2 receptors, a human CB2 cell-based assay was performed on CHO-K1cells. Data obtained after treatment with the formulation, single extracts and active compounds are summarized in Table 3; the CB2 agonist HU210 was tested in the same experiments as reference compound.
| Sample | Human CB2 cells | Mock cells | ||
|---|---|---|---|---|
| EC50 | Dose response curves | EC50 | Dose response curves | |
| Acmella oleracea | 12 µg/ml |  |
N.E. | 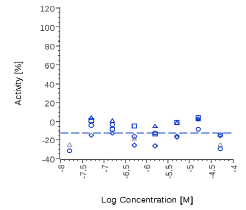 |
| Spilanthol | 3.66 µM | 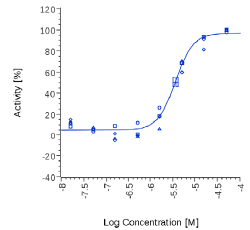 |
30.1 µM | 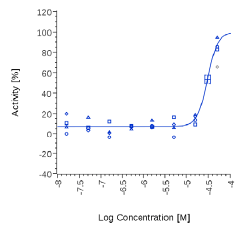 |
| Zingiber officinalis | N.E. | 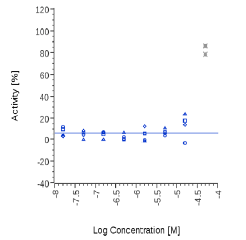 |
N.E. | 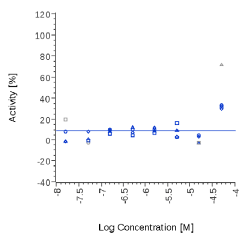 |
| 6-Gingerol | N.E. | 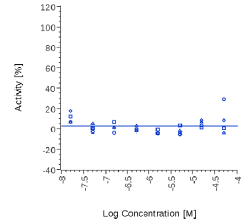 |
N.E. | 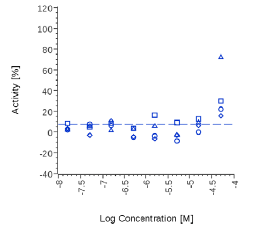 |
| Mitidol | 44.4 µg/ml | 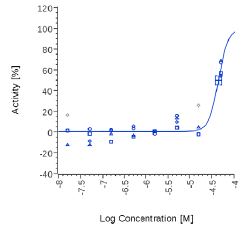 |
N.E. | 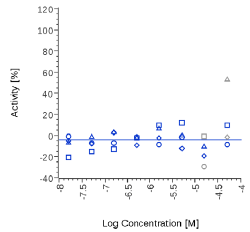 |
| HU210 | 1.35 nM | 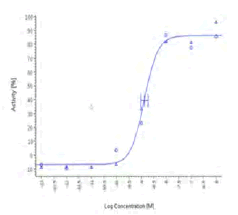 |
N.E. | 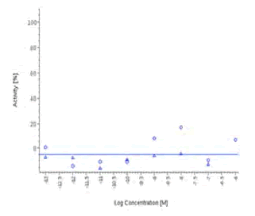 |
| Piper Nigrum | 2.43 µg/ml | 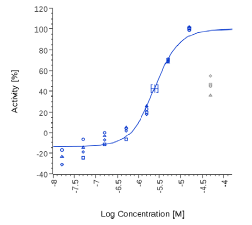 |
2.53 µg/ml | 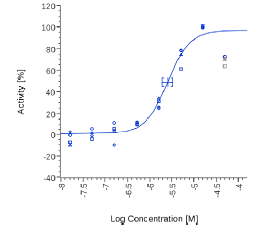 |
| Myrrh dry extract | N.E. | 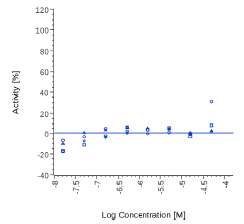 |
N.E. | 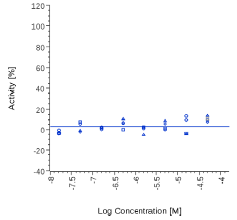 |
| PEA | N.E. | 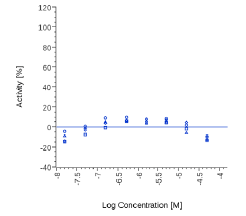 |
N.E. | 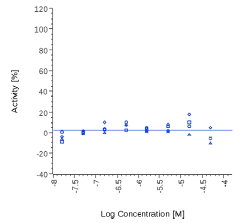 |
Table 3: Potency (EC50) on human CB2 cell based assay.
Mitidol, Acmella oleracea and Spilanthol showed an effect on human CB2 assay with EC50 values in the micromolar or μg/mL range. The effect of Mitidol and Acmella oleracea was not observed on the respective Mock cell line and appeared to be CB2 receptor-specific.
On the contrary, Spilanthol and Piper Nigrum were active in both human CB2 cells and Mock cells, suggesting an unspecific effect.
Zingiber officinale and 6-Gingerol showed no effects on that assay, as well as Myrrh dry extract and PEA, up to the maximal concentration tested (50 μg/ml). A positive effect was expected at least for PEA, which is reported to interact with other pathways/ mechanisms, like for example peroxisome proliferator-activated receptor PPAR [27].
Given current interest in the use of natural products with analgesic and anti-inflammatory properties, the present study was designed to explore the possible mechanism of action of the new food-grade formulation of two highly standardized botanical extracts, Zingiber officinale and Acmella oleracea, in preclinical endocannabinoid receptor models. The results, obtained in a panel of two in vitro assays, indicated an interesting effect of Mitidol on both FAAH inhibition and CB2 receptor agonism, partially explaining its oral use as a natural adjuvant in pain management.
It is well known that FAAH enzymes exist both in plants and animals. In plants FAAH inhibition induced stimulation of root growth [28], while in animals FAAH inhibition blocked the breakdown of the endogenous cannabinoid anandamide, with consequent prolonged cannabinoid receptor stimulation [29]. Therefore, the FAAH inhibition is considered as potential target for cannabinoid system modulation [30]. Several natural substances like N-isobutyl amides, particularly those from Echinacea spp, [31] have been studied both as activators of CB2 receptors and FAAH inhibitors [32,33].
N-isobutyl amides are also contained in Acmella oleracea, one of the extracts present in Mitidol. This paper reports for the first time that Acmella oleracea CO2 extract was able to inhibit FAAH with an IC50 of 28 ppm, and is also active in CB2 cell-based assay (EC50 12 μg/ ml), confirming its modulatory activity on the endocannabinoid system, both in an indirect (via FAAH inhibition) and direct (CB2 agonism) way. The involvement of the CB2 system in the effect of Acmella oleracea is especially intriguing, because CB2 receptor-mediated analgesic effects appeared mainly peripheral and devoid of the undesired CB1 receptors-related central effects [34]. Furthermore, recent evidence also suggested a CB2 protective role in neuroinflammation, in reducing proinflammatory factors and increasing anti-inflammatory cytokines [18].
Zingiber officinale extract did not directly activate the CB2 receptor, but its component 8-Gingerol is able to inhibit FAAH, more than 6- and 10-Gingerols, with 6-Shogaol being far more effective than Gingerols. The present data are in agreement with those reported by a Poster communication [35] for Zingiber: a weak CB receptor binding affinity and a strong FAAH inhibitory activity (particularly shown by 10-Shogaol). Very interestingly, 6-Shogaol appeared in our assay about three times more effective than the natural compound Kaempferol, known from literature as the most potent FAAH inhibitor among the commonly occurring flavonoids [26].
It was also reported that 6,8 and 10-Shogaol have higher antiinflammatory activity than the corresponding Gingerols in murine macrophage cell cultures by inhibiting the arachidonic acid release and nitric oxide synthesis [36]. Moreover, gingerols and especially 6-Shogaol displayed inhibitory activity on NLRP3 inflammasomemediated secretion of IL-1β in stimulated macrophages [37], extending the interest of these natural compounds, especially in inflammation.
From data obtained in the present experiments, Mitidol showed positive effects in both preclinical assays, demonstrating a biological interaction with CB2 receptors and FAAH enzyme activity. Overall, our results could support and possibly explain the positive data obtained in a clinical study performed on 50 subjects with moderate knee osteoarthritis: the 30-day supplementation with Mitidol produced significant improvements both on primary endpoints like Pain Intensity and Knee Function indexes, and on secondary outcomes as C-Reactive Protein (CRP) and ESR (Erythrocyte Sedimentation Rate) biomarkers, related to inflammation, together with a good safety profile demonstration [38].
In conclusion, these experiments suggest a strong rationale for the use of Mitidol, the Phytosome of Zingiber officinale (active in FAAH inhibition) and Acmella oleracea (active in FAAH inhibition and CB2 activation) as a natural adjuvant in pain management. Given the involvement of the endocannabinoid system in unhealthy conditions such as acute and chronic pain and inflammation, we can conclude that both FAAH inhibition and CB2 receptor agonism can contribute to the human efficacy of Mitidol, which additionally represents a very useful tool to orally deliver both Zingiber officinale and Acmella oleracea extracts.
We would like to express our gratitude to Dr. Paola Misiano for her valuable editorial support.
This work does not involve any human or animal studies.
Giovanna Petrangolini, Fabio Donzelli, Davide Berlanda, Pietro Allegrini and Antonella Riva are Indena’s employees. Indena performed the FAAH assay. Indena is proprietary of Mitidol. Axxam’s employees (Andrea Rossignoli and Michela Stucchi) performed the CB2 based assays on compounds provided and manufacturing by Indena. The research did not receive any specific grant from funding agencies in the public or not-for-profit sectors.
Citation: Petrangolini G, Donzelli F, Berlanda D, Allegrini P, Rossignoli A, Stucchi M, et al. (2020) Targeting Cannabinoid Receptors and Fatty Acid Amide Hydrolase: An Innovative Food-Grade Delivery System of Zingiber officinale and Acmella oleracea Extracts as Natural Adjuvant in Pain Management. J Nutr Food Sci 10:766. doi: 10.35248/2155-9600.20.10.766
Received: 27-Nov-2019 Accepted: 27-Dec-2019 Published: 03-Jan-2020 , DOI: 10.35248/2155-9600.20.10.1000766
Copyright: © 2019 Petrangolini G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.