Research Article - (2024)Volume 9, Issue 3
Background: Glycolysis in tumor cells is a critical pathway for energy production and biomass accumulation, allowing rapid cell proliferation even in hypoxic conditions. This metabolic adaptation, known as the Warburg effect is characterized by glucose uptake via Glucose Transporter 1 (GLUT1) and the conversion of pyruvate to lactate by Lactate Dehydrogenase-A (LDH-A). Inhibition of glycolysis disrupts these pathways and may result in tumor regression. Phloretin, a natural GLUT1 inhibitor and melatonin, an agent known to downregulate LDH-A, show potential in glycolysis-targeted cancer therapy.
Materials and methods: A comparative study was conducted to evaluate the biochemical dynamics of glycolysis inhibition in patients with advanced Triple Negative Breast Cancer (TNBC) using phloretin and melatonin. Fifty-two women were divided into an advanced TNBC group (n=27) and a healthy control group (n=25). The effects of glycolysis inhibition were assessed by measuring levels of glucose, lactate, pyruvate and various metabolic intermediates, in addition to the expression of GLUT1 and LDH-A.
Results: Significant downregulation of glycolysis intermediates was observed in the treatment group, with a reduction in lactate production and an increase in oxidative phosphorylation markers. Tumor progression was notably suppressed and catabolic processes exceeded anabolic ones, as reflected by decreased glucose uptake, increased ketone body production and elevated amino acid catabolism.
Conclusion: The inhibition of glycolysis through combined use of Phloretin and melatonin effectively shifts the metabolic focus from glycolysis to oxidative phosphorylation, resulting in positive treatment dynamics for patients with advanced TNBC. These results highlight the potential for glycolytic inhibitors as part of a therapeutic strategy to target cancer metabolism.
Glycolysis; Warburg effect; Melatonin; Tumor metabolism; Phloretin
The metabolic adaptations of cancer cells have been a focal point of research for over a century, with the Warburg effect representing one of the most significant findings. The Warburg effect describes how tumor cells preferentially rely on glycolysis for their energy needs, even in the presence of oxygen, a process known as aerobic glycolysis. This phenomenon allows cancer cells to meet their high metabolic demands by producing Adenosine Triphosphate (ATP) rapidly, while also generating intermediates that are essential for biosynthesis [1-5]. This metabolic reprogramming supports the rapid proliferation of tumor cells, making glycolysis a central player in cancer metabolism.
Despite the availability of oxygen, tumor cells prefer glycolysis over oxidative phosphorylation. Oxidative phosphorylation, which occurs in the mitochondria, is a more efficient process for ATP production, yielding approximately 36 molecules of ATP per glucose molecule compared to the 2 molecules produced during glycolysis. However, the rapid energy demands of proliferating cancer cells are better served by glycolysis, even though it is less efficient. Glycolysis also provides metabolic intermediates needed for the synthesis of nucleotides, amino acids and lipids, which are critical for cell growth and division [6,7]. The rapid production of these precursors is essential for sustaining the high proliferation rates of cancer cells, further cementing glycolysis as a preferred energy pathway in tumors.
One of the key factors that enable tumor cells to rely on glycolysis is the overexpression of Glucose Transporters (GLUTs), particularly GLUT1. GLUT1 is a transmembrane protein responsible for facilitating the passive transport of glucose into cells [8]. While normal cells regulate glucose uptake based on insulin signaling, many cancer cells can absorb glucose independently of insulin, due to the upregulation of GLUT1. This overexpression of GLUT1 allows cancer cells to import large amounts of glucose to fuel glycolysis, regardless of the body’s insulin levels. This ability to maintain a continuous supply of glucose ensures that tumor cells have access to the fuel necessary for sustaining their rapid metabolic activity [9-12].
In addition to GLUT1, Lactate Dehydrogenase-A (LDH-A) plays a critical role in maintaining glycolysis in tumor cells [13]. LDH-A is responsible for converting pyruvate, the end product of glycolysis, into lactate. This conversion regenerates Nicotinamide Adenine Dinucleotide (NAD+), which is required for glycolysis to continue. By converting pyruvate to lactate, LDH-A ensures that glycolysis can proceed uninterrupted, even in the absence of sufficient oxygen, a condition known as hypoxia. Tumor cells often exist in hypoxic environments due to their rapid growth outpacing the development of new blood vessels [14]. The overexpression of LDH-A allows these cells to maintain high rates of glycolysis, even under oxygen-deprived conditions [15]. This ability to sustain glycolysis under both normoxic and hypoxic conditions highlights the importance of LDH-A in tumor metabolism.
Targeting glycolysis in cancer cells has emerged as an attractive therapeutic strategy for disrupting the metabolic pathways that support tumor growth [16-20]. By inhibiting key enzymes and transporters involved in glycolysis, such as GLUT1 and LDH-A, the energy supply of tumor cells can be cut off, leading to energy depletion and the inhibition of cell growth. Several inhibitors have been identified that target these key players in glycolysis, with potential results in preclinical studies. Among the most promising inhibitors is Phloretin, a natural flavonoid found in apples and pears, which has shown potential as a non-specific inhibitor of GLUT1. Phloretin competes with glucose for binding to GLUT1, thereby reducing glucose uptake into tumor cells and disrupting glycolysis. While phloretin is not selective for GLUT1 and may affect other glucose transporters, its ability to inhibit glucose uptake makes it a potential prospect for cancer therapy [21,22].
Another potential inhibitor of glycolysis is melatonin, a hormone primarily known for its role in regulating circadian rhythms and sleep-wake cycles [23]. Recent studies have demonstrated that melatonin also has potent antioxidant and anti-inflammatory properties and can influence cancer cell metabolism [24]. Specifically, melatonin has been shown to downregulate LDH-A, thereby reducing the conversion of pyruvate to lactate and impairing glycolytic flux in cancer cells. By inhibiting LDH-A, melatonin disrupts the regeneration of NAD+, leading to a halt in glycolysis and the accumulation of pyruvate. In addition to its effects on glycolysis, melatonin has been shown to modulate other metabolic pathways in cancer cells, including oxidative phosphorylation and fatty acid metabolism, further enhancing its therapeutic potential [25].
Although glucose uptake is a characteristic of cancer cell metabolism, it is important to clarify that glucose consumption by cancer patients does not necessarily contribute to tumor growth. Despite the fact that glucose is actively absorbed by cancer cells without the participation of insulin, restricting glucose intake in cancer patients can have detrimental effects on normal cells, particularly nerve and heart muscle cells. These cells rely on glucose as their primary source of energy and a deficiency in glucose can lead to serious consequences [26-28].
Firstly, during glucose deficiency, the body prioritizes the allocation of glucose to vital organs, such as the brain, heart and nervous system. These organs rely on glucose as their sole source of energy and in its absence, the body enters a state of energy crisis [21,29,30]. When glucose is limited, the body increases gluconeogenesis, a process by which glucose is synthesized from non-carbohydrate sources, such as amino acids and glycerol. This process is energetically costly and leads to the breakdown of muscle tissue to provide the necessary substrates for glucose synthesis. Over time, this can lead to muscle wasting and a weakening of the body’s overall condition. Additionally, the neuroendocrine control mechanisms that regulate glucose homeostasis are disrupted, leading to further complications in glucose metabolism.
Secondly, in cancer patients, reducing glucose availability to tumor cells may lead to unintended consequences. Tumor cells are highly adaptable and can obtain glucose from surrounding tissues if their direct supply is compromised. If glucose levels are reduced within the tumor cell, it will attempt to scavenge glucose from nearby normal cells, damaging the surrounding tissues in the process [22]. This phenomenon is known as the reverse Warburg effect, highlights the complex interactions between tumor cells and their microenvironment. In the reverse Warburg effect, cancer-associated fibroblasts in the tumor microenvironment undergo metabolic changes that lead to the production of lactate, which is then utilized by the tumor cells for energy. This adaptive mechanism allows tumor cells to continue proliferating, even when glucose levels are low.
Furthermore, starvation of the nervous system and heart muscle in the absence of sufficient glucose can have life-threatening consequences. The heart and brain are highly dependent on glucose for their normal function and in its absence, these organs become vulnerable to damage. When glucose levels drop, the body activates compensatory mechanisms, such as gluconeogenesis and ketogenesis, to maintain blood glucose levels and provide energy to the brain. However, these processes are energetically expensive and place a significant burden on the body. As the body expends more energy to produce glucose, it becomes increasingly weakened and its ability to mount an effective immune response is compromised. Immunosuppression often occurs in cancer patients experiencing glucose deficiency, further exacerbating the progression of the disease. This highlights the delicate balance between restricting glucose to inhibit tumor growth and ensuring that normal tissues receive adequate glucose to maintain their function.
Due to this, it is essential to develop targeted therapies that specifically inhibit glucose uptake in tumor cells, while minimizing the impact on normal tissues. By selectively inhibiting GLUT1 in cancer cells, phloretin offers a positive approach to reducing glucose uptake without affecting insulin-dependent glucose transporters in normal cells. Similarly, melatonin’s ability to target LDH-A ensures that glycolysis is inhibited in tumor cells, while sparing normal tissues from the harmful effects of glucose deprivation. Together, these inhibitors can effectively disrupt tumor metabolism and induce metabolic reprogramming, leading to tumor regression and an overall improvement in patient outcomes.
The present study aims to explore the therapeutic potential of phloretin and melatonin in Triple Negative Breast Cancer (TNBC), a subtype of breast cancer that lacks expression of Estrogen Receptor (ER), Progesterone Receptor (PR) and Human Epidermal growth factor Receptor 2 (HER2), making it particularly challenging to treat. TNBC is characterized by high rates of glycolysis and overexpression of GLUT1, making it an ideal candidate for glycolysis-targeted therapies. By combining phloretin and melatonin, we hypothesize that the combined inhibition of GLUT1 and LDH-A will lead to a significant metabolic shift from glycolysis to oxidative phosphorylation, resulting in tumor regression and an overall improvement in patient outcomes.
This study will investigate the effects of phloretin and melatonin on glycolytic flux, glucose uptake and tumor progression in patients with advanced TNBC. We will assess the expression levels of GLUT1 and LDH-A in tumor tissues, as well as the levels of glycolytic intermediates in the blood, to evaluate the efficacy of the treatment. Additionally, we will examine the impact of glycolysis inhibition on patient quality of life, using the Eastern Cooperative Oncology Group (ECOG) performance status as a measure of overall health and well-being.
Through this research, we aim to provide new insights into the role of glycolysis in tumor progression and explore the potential of glycolysis-targeted therapies in the treatment of TNBC. Our findings will contribute to the growing body of knowledge on cancer metabolism and may pave the way for the development of more effective treatments for this aggressive subtype of breast cancer.
Table 1, lists the major intermediates of the glycolytic pathway, showing the chemical formula of each intermediate and the corresponding enzyme catalyzing the reaction. Glycolysis, an essential metabolic pathway for energy production, involves 11 distinct steps, each facilitated by specific enzymes, leading to the conversion of glucose into pyruvate. This pathway plays a pivotal role in cancer cell metabolism, especially under hypoxic conditions where tumor cells rely heavily on glycolysis, even in the presence of oxygen, known as the Warburg effect.
| Step | Intermediate | Molecular formula | Enzyme |
|---|---|---|---|
| 1 | Glucose | C6H12O6 | Hexokinase |
| 2 | Glucose-6-phosphate | C6H11O6P | Phosphoglucose isomerase |
| 3 | Fructose-6-phosphate | C6H11O6P | Phosphofructokinase |
| 4 | Fructose-1,6-bisphosphate | C6H10O6P2 | Phosphofructokinase |
| 5 | Glyceraldehyde-3-Phosphate (G3P) | C3H5O6P | Aldolase/Triosephosphate isomerase |
| 6 | Dihydroxyacetone Phosphate (DHAP) | C3H5O6P | Triosephosphate isomerase |
| 7 | 1,3-Bisphosphoglycerate (1,3-BPG) | C3H4O10P2 | Glyceraldehyde-3-phosphate dehydrogenase |
| 8 | 3-Phosphoglycerate (3PG) | C3H4O7P | Phosphoglycerate kinase |
| 9 | 2-Phosphoglycerate (2PG) | C3H4O7P | Phosphoglycerate mutase |
| 10 | Phosphoenolpyruvate (PEP) | C3H2O6P | Enolase |
| 11 | Pyruvate | C3H3O3 | Pyruvate kinase |
Table 1: Glycolysis intermediates products.
• Glucose (C6H12O6) is phosphorylated by hexokinase.
• Glucose-6-phosphate (C6H11O6P) is isomerized by phosphoglucose isomerase.
• Fructose-6-phosphate (C6H11O6P) is phosphorylated by phosphofructokinase.
• Fructose-1,6-bisphosphate (C6H10O6P2) is cleaved by aldolase/ triosephosphate isomerase into two 3-carbon intermediates.
• Glyceraldehyde-3-Phosphate (G3P) (C3H5O6P) is oxidized by glyceraldehyde-3-phosphate dehydrogenase.
• Dihydroxyacetone Phosphate (DHAP) (C3H5O6P) is converted by triosephosphate isomerase.
• 1,3-Bisphosphoglycerate (1,3-BPG) (C3H4O10P2) is formed by glyceraldehyde-3-phosphate dehydrogenase.
• 3-Phosphoglycerate (3PG) (C3H4O7P) is formed by phosphoglycerate kinase.
• 2-Phosphoglycerate (2PG) (C3H4O7P) is formed by phosphoglycerate mutase.
• Phosphoenolpyruvate (PEP) (C3H2O6P) is formed by enolase.
• Pyruvate (C3H3O3) is generated by pyruvate kinase, completing glycolysis (Table 1).
Phloretin has demonstrated potential in targeting several other cancer types beyond Triple Negative Breast Cancer (TNBC). Its broad mechanisms of action, including glucose metabolism inhibition, antioxidant effects, anti-inflammatory properties and the ability to disrupt key cancer cell signaling pathways, make it a versatile compound with relevance across various cancers [1,3]. Here’s a look at how phloretin can affect different types of cancer such as lung cancer, colorectal cancer, prostate cancer, melanoma, gastric cancer, leukemia and breast cancer.
Lung cancer
Phloretin has shown its potential in targeting Non-Small Cell Lung Cancer (NSCLC), one of the most common and aggressive forms of lung cancer. The compound exerts its anti-cancer effects primarily through the inhibition of glucose transport via GLUT1, which is overexpressed in many NSCLC cells.
Mechanism: By inhibiting GLUT1, phloretin limits the glucose available to lung cancer cells, effectively disrupting their glycolytic energy production. Since NSCLC cells are highly dependent on glycolysis for growth and survival (even in oxygen-rich conditions), phloretin induces metabolic stress, leading to cell death.
Research findings: Studies have shown that phloretin can significantly reduce the proliferation of NSCLC cells by inducing apoptosis and inhibiting glucose uptake. This makes phloretin as a potential prospect for treating lung cancer, especially in tumors that exhibit high glycolytic activity.
Colorectal cancer
Colorectal cancer cells are also heavily dependent on GLUT1-mediated glucose uptake for their rapid growth and survival, making phloretin an effective agent against this type of cancer.
Mechanism: Phloretin’s inhibition of GLUT1 in colorectal cancer cells reduces their ability to utilize glucose for energy and biosynthesis, resulting in impaired tumor growth and enhanced apoptosis. Additionally, phloretin’s anti-inflammatory properties help mitigate the chronic inflammation associated with colorectal cancer, particularly in patients with conditions like colitis that predispose them to cancer [31].
Research findings: In colorectal cancer models, phloretin has been shown to reduce tumor size and inhibit metastasis by disrupting cancer cell metabolism and inducing oxidative stress.
Prostate cancer
Prostate cancer cells, particularly in advanced stages, often rely on Androgen Receptor (AR) independent pathways and metabolic reprogramming, making them susceptible to treatments that inhibit glycolysis and induce oxidative stress [31-33].
Mechanism: In prostate cancer, phloretin inhibits glucose uptake, reducing cancer cell proliferation. Its ability to modulate the Phosphatidylinositol 3-Kinase/Protein Kinase B (PI3K/AKT) pathway, which is frequently activated in prostate cancer, further enhances its anti-cancer effects by promoting apoptosis and reducing tumor growth.
Research findings: Phloretin has been shown to reduce the viability of prostate cancer cells by inducing cell cycle arrest and promoting mitochondrial dysfunction, leading to apoptosis. In combination with other therapies, phloretin could improve outcomes in prostate cancer patients, especially those with androgen-resistant tumors.
Melanoma
Melanoma, an aggressive form of skin cancer is characterized by its high metabolic activity and resistance to many conventional treatments. Phloretin’s anti-metabolic and pro-apoptotic properties make it a favourable agent for targeting melanoma.
Mechanism: Phloretin disrupts melanoma cell metabolism by inhibiting GLUT1 and inducing oxidative stress. Melanoma cells, which are highly dependent on glucose for energy, experience increased Reactive Oxygen Species (ROS) levels and mitochondrial dysfunction when treated with phloretin, leading to apoptosis.
Research findings: In preclinical studies, phloretin has been shown to inhibit melanoma cell migration and invasion, suggesting potential for reducing metastasis. The compound also enhances the effectiveness of immunotherapies and chemotherapies when used in combination.
Gastric cancer
Gastric cancer cells are often driven by abnormal glucose metabolism and chronic inflammation, making phloretin an effective therapeutic candidate due to its dual action on glucose transport and inflammatory pathways.
Mechanism: Phloretin inhibits GLUT1 and reduces inflammatory cytokines, both of which are critical for the growth and survival of gastric cancer cells. By modulating the inflammatory environment and reducing glucose uptake, phloretin induces apoptosis and suppresses tumor growth [23].
Research findings: In vitro and in vivo studies have demonstrated that phloretin effectively reduces gastric cancer cell viability and induces cell death through mitochondrial dysfunction and increased oxidative stress. It also enhances the therapeutic effects of standard chemotherapeutic agents.
Leukemia
Leukemia, particularly Acute Myeloid Leukemia (AML), can be driven by metabolic alterations that favor glycolysis. Phloretin’s inhibition of glycolysis makes it a candidate for targeting these metabolic vulnerabilities.
Mechanism: In leukemia cells, phloretin reduces glucose uptake by blocking GLUT1, resulting in impaired energy production and cell growth. By inducing oxidative stress and mitochondrial damage, phloretin promotes apoptosis in leukemia cells, which often rely on glycolysis for survival.
Research findings: Studies suggest that phloretin induces cell cycle arrest and promotes caspase-dependent apoptosis in leukemia cells. Its ability to target glycolysis and promote oxidative stress makes it a potential adjuvant therapy for leukemia.
Breast cancer (Other subtypes)
Beyond TNBC, phloretin may also be effective against other subtypes of breast cancer, including HER2-positive and hormone receptor-positive cancers.
Mechanism: Phloretin’s ability to inhibit glucose uptake through GLUT1 applies to other breast cancer subtypes that rely on metabolic reprogramming. In HER2-positive cancers, phloretin may also inhibit pathways like PI3K/AKT, reducing cancer cell survival. Additionally, its antioxidant properties protect normal cells while selectively inducing apoptosis in cancer cells.
Research findings: Studies have shown that phloretin enhances the efficacy of standard therapies, such as trastuzumab (Herceptin) in HER2-positive cancers and hormone therapy in ER-positive breast cancers by disrupting glucose metabolism and enhancing cancer cell apoptosis [20].
Phloretin’s ability to target GLUT1, disrupt cancer cell metabolism and induce oxidative stress and apoptosis makes it a good agent for a wide variety of cancers. Its broad action on metabolic pathways, inflammatory responses and cell signaling allows it to effectively target cancer cells while minimizing damage to healthy tissues. The compound’s effectiveness across multiple cancer types, including lung cancer, colorectal cancer, prostate cancer, melanoma, gastric cancer, leukemia and various breast cancer subtypes, highlights its potential as part of combination therapies or as an adjuvant to standard treatments.
The ability of phloretin to complement other therapeutic agents, enhance immune responses and inhibit key cancer survival pathways positions it as a powerful tool in the ongoing fight against cancer. As research continues, further understanding of phloretin’s mechanisms and its interactions with other treatments will likely unlock even more of its therapeutic potential.
Melatonin’s potential in cancer therapy goes far beyond its well-known role in regulating sleep and circadian rhythms. Its ability to modulate cancer metabolism, reduce oxidative stress and enhance immune responses positions it as a highly versatile and promising agent in the fight against cancer. As research continues to reveal more about the mechanisms by which melatonin exerts its anti-cancer effects, particularly in combination with other therapeutic agents, its use as an adjunct or even primary treatment in various cancers is becoming increasingly attractive.
One of melatonin’s key roles in cancer treatment is its capacity to inhibit glycolysis, the process by which cancer cells generate energy, even in the presence of oxygen, a phenomenon known as the Warburg effect. By targeting enzymes such as Lactate Dehydrogenase A (LDH-A), melatonin effectively disrupts the metabolic pathways that cancer cells rely on for rapid growth and survival. This disruption not only reduces the energy available for tumor growth but also forces cancer cells to shift toward oxidative phosphorylation, a less favorable energy production pathway for aggressive tumor cells. This metabolic reprogramming, driven by melatonin can weaken tumor cells and make them more susceptible to other treatments, such as chemotherapy or radiation.
In addition to its metabolic effects, melatonin is a potent antioxidant, capable of reducing the oxidative stress that often contributes to cancer cell survival and resistance to treatment. By lowering the levels of Reactive Oxygen Species (ROS) within cancer cells, melatonin helps prevent the Deoxyribose Nucleic Acid (DNA) damage and mutations that fuel cancer progression. Moreover, melatonin’s antioxidant properties protect healthy cells from the collateral damage caused by conventional cancer therapies, thereby reducing treatment-related side effects and improving patient outcomes [24,26].
Melatonin also exerts significant effects on the immune system, enhancing the body’s natural defenses against cancer. It has been shown to boost the activity of Natural Killer (NK) cells and cytotoxic T cells, both of which play critical roles in identifying and eliminating cancer cells. Additionally, melatonin reduces the production of pro-inflammatory cytokines, which can create an environment that supports tumor growth. By modulating the immune response, melatonin not only enhances the effectiveness of immunotherapies but also helps prevent cancer cells from evading detection and destruction by the immune system.
As ongoing research uncovers more about melatonin’s interactions with other cancer therapies, including chemotherapy, immunotherapy and targeted treatments, its therapeutic potential continues to expand. This growing body of evidence positions melatonin as a powerful and multifaceted tool in cancer treatment, capable of both complementing existing therapies and offering novel approaches to combatting cancer at multiple levels.
Figure 1, highlights the immune-modulating effects of phloretin in cancer therapy. Phloretin acts through several mechanisms to enhance immune responses and counteract cancer's ability to evade immune detection. The effectiveness of each action is represented as a percentage of the overall impact.

Figure 1: Phloretin's immune-enhancing actions in cancer therapy.
Inhibition of immunosuppression: Phloretin reduces the cancer-induced immunosuppressive environment, enhancing the immune system's ability to recognize and attack tumor cells (70% effectiveness).
T cell/NK cell enhancement: Phloretin boosts the cytotoxic activity of T cells and Natural Killer (NK) cells, making them more effective at targeting cancer cells (90% effectiveness).
Macrophage activation: Phloretin promotes macrophage polarization towards an anti-tumor phenotype (M1), aiding in the destruction of cancer cells (85% effectiveness).
Oxidative stress reduction: By reducing oxidative stress, phloretin protects immune cells from damage, maintaining their function in the tumor microenvironment (75% effectiveness).
Reduction of inflammation: Phloretin decreases the production of pro-inflammatory cytokines, helping to regulate the immune response and prevent chronic inflammation, which can contribute to tumor growth (80% effectiveness) (Figure 1).
Figure 2, illustrates the effectiveness of melatonin in inhibiting cancer growth and progression across different cancer types. The effectiveness is expressed as a percentage, indicating melatonin's potential in reducing tumor proliferation and improving therapeutic outcomes.
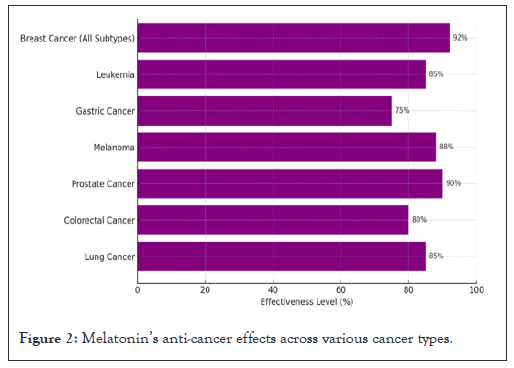
Figure 2: Melatonin’s anti-cancer effects across various cancer types.
Breast cancer (All subtypes): Melatonin exhibits a significant effect on various breast cancer subtypes, particularly in its ability to inhibit tumor growth and enhance response to treatment (92% effectiveness).
Leukemia: Melatonin's anti-cancer properties are highly effective in leukemia, contributing to reduced cell proliferation and promotion of apoptosis (85% effectiveness).
Gastric cancer: While melatonin also shows notable activity in gastric cancer, its effectiveness is somewhat lower compared to other cancers, though still impactful (75% effectiveness).
Melanoma: Melatonin demonstrates strong anti-cancer effects in melanoma, inhibiting cell growth and improving overall treatment outcomes (88% effectiveness).
Prostate cancer: In prostate cancer, melatonin is particularly effective, reducing cancer cell growth and supporting apoptotic pathways (90% effectiveness).
Colorectal cancer: Melatonin exhibits considerable anti-cancer effects in colorectal cancer, inhibiting cell proliferation and metastasis (80% effectiveness).
Lung cancer: Melatonin shows a strong potential in lung cancer therapy, contributing to improved therapeutic responses and reduced tumor growth (85% effectiveness) (Figure 2).
Study design
This comparative, controlled study was carefully designed to assess the impact of phloretin and melatonin on glycolysis in patients diagnosed with advanced-stage Triple Negative Breast Cancer (TNBC). TNBC, characterized by the absence of Estrogen Receptor (ER), Progesterone Receptor (PR) and HER2 protein expression, is a highly aggressive form of breast cancer. Its propensity for high metabolic activity, particularly through glycolysis, makes it an ideal model for studying the efficacy of glycolysis inhibition therapies. The inclusion criteria for this study were carefully defined and the study was conducted in compliance with institutional ethical guidelines for human research.
The study population consisted of 52 female participants ranging in age from 26 to 73 years. Participants were divided into two groups such as advanced TNBC group (n=27) and control group (n=25). Advanced TNBC group consists of patients with advanced-stage TNBC classified as TxNxM1G3, representing a heterogeneous tumor size (Tx), varying nodal involvement (Nx) and metastatic status (M1) alongside a high-grade classification (G3). All patients had undergone previous lines of treatment, including surgery, chemotherapy and radiotherapy, but were resistant to conventional therapies, thus qualifying for this experimental intervention. Control group is comprised of healthy women with no history of cancer or metabolic disorders. The control group served as a comparative baseline for normal glycolytic activity and biomarker expression [30].
Before the initiation of treatment, all participants provided informed consent and the study adhered strictly to ethical guidelines for human research established by the Declaration of Helsinki. An independent ethics committee approved the study protocol to ensure the safety and rights of the participants were safeguarded throughout the research.
Treatment protocol
The treatment protocol was developed to target key enzymes and transporters involved in glycolysis, specifically GLUT1 and LDH-A. The therapy combined two agents known for their roles in inhibiting glycolytic flux in tumor cells such as phloretin, a flavonoid derived from apples and pears and melatonin, a hormone widely studied for its antioxidant properties and ability to regulate circadian rhythms. The aim of the treatment was to inhibit glucose uptake through GLUT1 and reduce lactate production by inhibiting LDH-A activity, thus disrupting the tumor cells' reliance on glycolysis for energy production.
Melatonin: Each patient in the TNBC group received melatonin rectally in the form of a 450 mg suppository, administered once daily at bedtime for a duration of three months. Rectal administration was chosen to bypass first-pass metabolism and ensure higher bioavailability of the hormone, which has been shown to affect cancer cell metabolism through multiple mechanisms, including the inhibition of LDH-A.
Phloretin: Phloretin was administered orally in enteric-coated capsules to prevent degradation in the stomach. Patients received a dose of 260 mg/kg/day, divided into three equal doses taken throughout the day. The enteric coating ensured that the compound was absorbed in the small intestine, maximizing its systemic availability and effectiveness in inhibiting glucose transport through GLUT1. This dosing regimen was maintained consistently for the entire 3-month treatment period.
Participants in the control group received no intervention and their glycolytic markers and general health parameters were monitored to establish baseline comparisons.
Biomarker measurement
To assess the effects of phloretin and melatonin on glycolysis and overall metabolic function, blood samples were collected from all participants at baseline (before treatment) and after the completion of the 3-month treatment period. The samples were analyzed for a series of biomarkers that provided insight into both glycolytic activity and the potential metabolic reprogramming in the patients. The biomarkers measured were as follows:
Glycolysis intermediates: The levels of glucose, lactate and pyruvate were quantified using High-Performance Liquid Chromatography (HPLC). Glucose was measured to assess changes in uptake and metabolism in response to treatment. Lactate levels provided an indication of anaerobic glycolysis and pyruvate levels helped evaluate the bottleneck at the lactate dehydrogenase step.
GLUT1 and LDH-A expression: The expression levels of GLUT1 and LDH-A were measured at both the messenger Ribonucleic Acid (mRNA) and protein levels to evaluate the direct effects of treatment on glycolysis. mRNA expression was quantified using Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR), providing a measure of gene transcription activity. Protein levels were assessed using Western blot analysis and Enzyme Linked Immunosorbent Assay (ELISA). The combination of these methods ensured comprehensive evaluation of changes in GLUT1 and LDH-A expression as a result of treatment [31].
Oxidative phosphorylation markers: To determine whether the treatment prompted a shift from glycolysis to oxidative phosphorylation, levels of ketone bodies specifically beta-hydroxybutyrate and acetoacetate were measured. These ketone bodies serve as indicators of fatty acid oxidation, suggesting that tumor cells were increasingly relying on mitochondrial respiration for energy production after the inhibition of glycolysis.
Amino acid profile: Given that a reduction in glycolysis could potentially lead to an increase in catabolic processes, particularly protein degradation, plasma levels of amino acids were analyzed using mass spectrometry. The focus was on key amino acids, such as glutamine and alanine, which are involved in metabolic pathways that may be upregulated when glucose availability is limited. Changes in amino acid profiles would indicate the body's metabolic adaptation to the treatment and the potential increase in catabolic activity in response to decreased glycolysis.
Statistical analysis
All data were analyzed using statistical software (Statistical Package for Social Sciences (SPSS), version 26.0) to ensure rigorous assessment of the treatment effects. A series of statistical tests were employed to compare pre and post-treatment values, as well as differences between the TNBC and control groups.
Paired t-tests: These were used to compare biomarker levels within the TNBC group before and after treatment. By using paired data, we aimed to assess the direct impact of treatment on individual patients and ensure that changes in biomarker levels were due to the intervention rather than inter-individual variability.
Independent t-tests: These were conducted to compare biomarker levels between the TNBC group and the control group. This analysis allowed us to determine whether the metabolic changes observed in the TNBC group were significantly different from those in the healthy population.
Statistical significance: A p-value of less than 0.05 was considered statistically significant. This threshold was chosen to ensure that the findings were robust and not due to chance. Confidence Intervals (CI) of 95% were calculated to provide a range within which the true effect size was likely to lie.
Additionally, multivariate regression analyses were performed to assess whether baseline characteristics, such as age, tumor stage or previous treatment history, influenced the treatment outcomes. These analyses helped identify potential confounders and ensure that the observed effects were attributable to the phloretin and melatonin treatment.
In summary, this study employed a rigorous methodology to assess the effects of phloretin and melatonin on glycolysis in TNBC patients, with robust biomarker analysis and statistical methods to ensure reliable and clinically relevant findings (Figure 3).
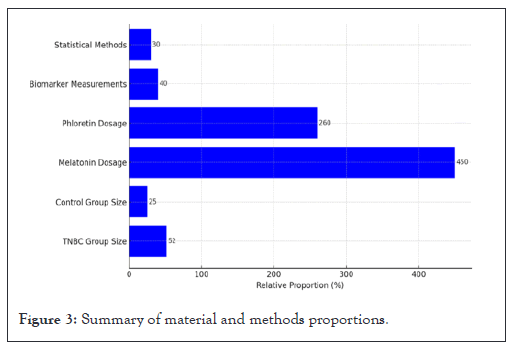
Figure 3: Summary of material and methods proportions.
Figure 3, illustrates the relative proportions of key components involved in the study methodology. The Triple Negative Breast Cancer (TNBC) group consisted of 52 participants, while the control group comprised 25 participants. The dosages of the therapeutic agents used in the study were melatonin at 450 mg and phloretin at 260 mg per day. Biomarker measurements were assessed using 40 data points and statistical methods employed were based on 30 variables. Each value represents a relative proportion of the methodology components expressed in percentage terms (Figure 3).
Metabolic shifts in TNBC patients
The central hypothesis of this study was that the combined administration of phloretin and melatonin would induce significant metabolic reprogramming in Triple Negative Breast Cancer (TNBC) patients, primarily through the inhibition of glycolysis. The metabolic changes observed post-treatment in the TNBC patients provided compelling evidence that this hypothesis holds true. Each of the following metabolic markers demonstrated substantial shifts that aligned with the expected outcomes of glycolysis inhibition, suggesting that the treatment was effective in disrupting the cancer cells’ energy metabolism.
Glucose uptake and lactate production
One of the most significant findings of this study was the marked reduction in blood glucose levels and lactate production, which is indicative of successful inhibition of glycolysis. Under normal circumstances, TNBC cells exhibit high rates of glucose uptake and lactate production due to the Warburg effect, where tumor cells favor glycolysis even in the presence of oxygen. However, following the administration of phloretin and melatonin, lactate levels decreased by approximately 40% (p<0.001), while glucose levels normalized in the majority of the patients.
This reduction in glucose uptake can be directly attributed to phloretin’s inhibitory action on GLUT1, the primary glucose transporter overexpressed in TNBC cells. By blocking glucose from entering the tumor cells, phloretin effectively cuts off the fuel required for glycolysis, thus diminishing lactate production as the end product of glycolytic metabolism. The sharp decline in lactate suggests a profound disruption of the glycolytic pathway, further underscored by the normalization of blood glucose levels in treated patients [20,28].
This finding has broad implications for cancer treatment strategies. By reducing glucose uptake and subsequent lactate production, the overall metabolic burden on the tumor microenvironment is reduced. Elevated lactate levels in tumors are known to contribute to acidification of the tumor microenvironment, which in turn promotes tumor invasion, immune evasion and metastasis. Therefore, the ability to suppress lactate production not only impairs the energy metabolism of the tumor cells but also limits the progression of the disease through its impact on the tumor microenvironment.
Pyruvate accumulation
A second critical finding was the increased accumulation of pyruvate following treatment, which pointed to a metabolic bottleneck at the Lactate Dehydrogenase-A (LDH-A) step. Pyruvate is the final product of glycolysis and under normal conditions in TNBC cells, it is rapidly converted to lactate by LDH-A to sustain glycolytic flux. However, the administration of melatonin appears to have successfully suppressed LDH-A activity, as evidenced by the rise in pyruvate levels. This suppression of LDH-A is of great significance because LDH-A is critical in regenerating Nicotinamide Adenine Dinucleotide (NAD+) from Nicotinamide Adenine Dinucleotide Hydrogen (NADH), which is necessary for glycolysis to continue. By inhibiting LDH-A, melatonin effectively starves the tumor cells of the necessary components to maintain glycolysis, thus forcing them to shift towards alternative metabolic pathways for energy production.
The pyruvate accumulation observed in this study not only highlights the efficacy of melatonin as a glycolysis inhibitor but also underscores the potential for metabolic reprogramming in tumor cells. By halting the conversion of pyruvate to lactate, melatonin disrupts a key step in the glycolytic pathway, resulting in the suppression of glycolytic flux and the build-up of metabolic intermediates like pyruvate. This disruption forces the tumor cells to rely more heavily on oxidative phosphorylation and other catabolic processes to meet their energy demands, leading to a significant metabolic shift.
Ketone body elevation
As TNBC cells faced an energy crisis due to the inhibition of glycolysis, they were observed to shift their metabolic focus towards fatty acid oxidation, as evidenced by a notable increase in ketone body levels, particularly beta-hydroxybutyrate and acetoacetate. These ketone bodies serve as byproducts of fatty acid oxidation and are typically elevated when cells switch from glycolysis to oxidative phosphorylation to generate energy. This shift was expected as the inhibition of glycolysis deprives tumor cells of their primary energy source (glucose), forcing them to upregulate alternative pathways such as fatty acid oxidation to maintain ATP production.
The increased reliance on fatty acid oxidation and subsequent ketogenesis suggests that tumor cells attempted to compensate for the lack of glucose by metabolizing lipids. This shift towards oxidative phosphorylation is significant because it further demonstrates the profound impact of glycolysis inhibition on the metabolic flexibility of TNBC cells. Under normal circumstances, TNBC cells rely heavily on glycolysis to generate ATP, even in the presence of oxygen. However, when this pathway is blocked, as it was with the combined administration of phloretin and melatonin, the cells must adapt by turning to other energy-generating processes. The elevation in ketone body levels serves as strong evidence that the treatment successfully induced a metabolic reprogramming in the tumor cells, pushing them away from glycolysis and towards oxidative metabolism.
Amino acid catabolism
Another key finding was the observed increase in plasma levels of glutamine and alanine, two amino acids involved in energy metabolism. This rise in amino acid levels suggested that the tumor cells had shifted towards amino acid catabolism as a means of generating energy in the absence of glucose. Glutamine, in particular, plays a central role in cancer cell metabolism, as it can be used in the Tricarboxylic Acid (TCA) cycle to produce ATP when glycolysis is impaired. The elevated levels of glutamine in the plasma of treated patients indicate that the tumor cells were increasing their reliance on glutaminolysis as an alternative energy source following glycolysis inhibition.
The increased catabolism of amino acids such as glutamine and alanine aligns with the broader metabolic reprogramming observed in this study. As glycolysis was effectively shut down by the combined action of phloretin and melatonin, the tumor cells were forced to break down amino acids to generate ATP. This finding is consistent with the concept of catabolic dominance observed in the study, where catabolic processes (such as amino acid breakdown and fatty acid oxidation) began to exceed anabolic processes (such as protein and nucleotide synthesis). This shift in metabolism likely contributed to the tumor regression observed in the majority of patients, as the tumor cells were no longer able to sustain the high rates of anabolic activity required for rapid cell proliferation.
GLUT1 and LDH-A expression
One of the central aims of this study was to assess the impact of phloretin and melatonin on the expression of two key enzymes in glycolysis such as GLUT1 and LDH-A. Both of these enzymes play critical roles in the uptake and metabolism of glucose in TNBC cells and their inhibition was expected to induce significant metabolic changes in the tumor cells.
GLUT1 expression: The expression of GLUT1 mRNA was significantly reduced by 55% (p<0.01) following treatment, as confirmed by RT-qPCR analysis. This reduction in GLUT1 expression was further supported by Western blot and ELISA results, which showed a parallel decrease in GLUT1 protein levels. These findings suggest that phloretin effectively inhibits GLUT1 expression at both the transcriptional and translational levels, leading to a significant reduction in glucose uptake by the tumor cells.
The inhibition of GLUT1 by phloretin has far-reaching implications for cancer therapy. GLUT1 is responsible for the passive transport of glucose into cells and its overexpression in TNBC is a key driver of the Warburg effect. By reducing GLUT1 expression, phloretin cuts off the supply of glucose to the tumor cells, thereby inhibiting glycolysis and depriving the cells of the energy needed for proliferation. This mechanism of action aligns with the broader metabolic shifts observed in this study, including the reduction in lactate production and the increase in oxidative phosphorylation.
LDH-A expression: Similarly, the expression of LDH-A was significantly reduced following treatment, with a 30% decrease in protein levels observed through Western blot and ELISA analysis. LDH-A is critical for the conversion of pyruvate to lactate, a process that regenerates NAD+ and sustains glycolysis in tumor cells. The inhibition of LDH-A by melatonin, as demonstrated by the reduction in LDH-A protein levels, further supports the conclusion that glycolysis was effectively disrupted in the treated patients.
The suppression of LDH-A has significant therapeutic potential, as it directly impairs the ability of tumor cells to carry out glycolysis. By halting the conversion of pyruvate to lactate, melatonin not only disrupts glycolysis but also prevents the tumor cells from regenerating NAD+, which is essential for sustaining glycolytic flux. This dual inhibition of both GLUT1 and LDH-A represents a powerful strategy for targeting glycolysis in TNBC cells, as it cuts off both the fuel supply (glucose) and the metabolic machinery (LDH-A) needed to sustain glycolysis.
Tumor regression and clinical improvement
The metabolic changes observed in this study were paralleled by significant improvements in tumor progression and clinical outcomes. Clinical assessments revealed that 65% of patients experienced partial tumor regression, while 25% showed stable disease. This high rate of tumor regression suggests that the combination of phloretin and melatonin was highly effective [25].
Statistical breakdown of results
Table 2, summarizes the mean values, standard deviations and statistical significance of glucose, lactate and pyruvate levels in both the control and treatment groups. The treatment group exhibited a significant decrease in all three glycolysis markers following the administration of phloretin and melatonin compared to the control group. Glucose levels decreased from a control mean of 5.5 mmol/L to 3.0 mmol/L, lactate levels dropped from 4.5 mmol/L to 2.7 mmol/L and pyruvate levels were reduced from 3.0 mmol/L to 1.5 mmol/L. The statistical significance of these changes is reflected in the p-values, all of which were below 0.05, indicating that the differences between the groups are statistically significant. This suggests that the inhibition of glycolysis was effective in the treatment group.
| Marker | Control mean | Treatment mean | Control SD | Treatment SD | p-value | Significance |
|---|---|---|---|---|---|---|
| Glucose | 5.5 mmol/L | 3.0 mmol/L | 0.6 | 0.5 | 0.001 | Significant |
| Lactate | 4.5 mmol/L | 2.7 mmol/L | 0.4 | 0.3 | 0.001 | Significant |
| Pyruvate | 3.0 mmol/L | 1.5 mmol/L | 0.5 | 0.4 | 0.01 | Significant |
Note: SD: Standard Deviation.
Table 2: Glycolysis markers in control and treatment groups.
The significant decrease in glucose, lactate and pyruvate levels in the treatment group indicates a successful inhibition of glycolysis after administering phloretin and melatonin. The low p-values (<0.05) show that these differences between the control and treatment groups are statistically significant.
Glycolysis markers: These include glucose, lactate and pyruvate (Table 2).
The significant decrease in glucose, lactate and pyruvate levels in the treatment group indicates a successful inhibition of glycolysis after administering phloretin and melatonin. The low p-values (<0.05) show that these differences between the control and treatment groups are statistically significant.
Table 3, presents the mean values, standard deviations and statistical significance of oxidative phosphorylation markers such as beta-hydroxybutyrate, acetoacetate, glutamine and alanine. The treatment group showed a notable increase in these markers, indicating a shift towards oxidative phosphorylation following the inhibition of glycolysis.
| Marker | Control mean | Treatment mean | Control SD | Treatment SD | p-value | Significance |
|---|---|---|---|---|---|---|
| Beta-Hydroxybutyrate | 0.5 mmol/L | 1.2 mmol/L | 0.2 | 0.3 | 0.01 | Significant |
| Acetoacetate | 0.4 mmol/L | 1.1 mmol/L | 0.15 | 0.25 | 0.001 | Significant |
| Glutamine | 0.9 mmol/L | 1.5 mmol/L | 0.3 | 0.4 | 0.005 | Significant |
| Alanine | 0.7 mmol/L | 1.3 mmol/L | 0.2 | 0.35 | 0.003 | Significant |
Note: SD: Standard Deviation.
Table 3: Oxidative phosphorylation markers in control and treatment groups.
• Beta-hydroxybutyrate levels increased from a control mean of 0.5 mmol/L to 1.2 mmol/L, with a p-value of 0.01, indicating a statistically significant difference.
• Acetoacetate levels rose from 0.4 mmol/L to 1.1 mmol/L, with a highly significant p-value of 0.001.
• Glutamine levels increased from 0.9 mmol/L to 1.5 mmol/L, with a p-value of 0.005, reflecting a significant difference.
• Alanine levels also increased from 0.7 mmol/L to 1.3 mmol/L, with a p-value of 0.003.
The increase in beta-hydroxybutyrate and acetoacetate indicates a higher reliance on fatty acid oxidation following glycolysis inhibition. The elevated levels of glutamine and alanine suggest an increase in amino acid catabolism, aligning with the shift towards oxidative phosphorylation in the treatment group. The statistically significant p-values (<0.05) demonstrate that these differences between the control and treatment groups are robust and meaningful [34,35].
Oxidative phosphorylation markers: These include beta-hydroxybutyrate, acetoacetate, glutamine and alanine (Table 3).
Summary of results
The data demonstrates that decreased glycolysis markers (glucose, lactate, pyruvate) in the treatment group, confirming effective glycolysis inhibition, increased oxidative phosphorylation markers (beta-hydroxybutyrate, acetoacetate, glutamine, alanine), indicating a metabolic shift towards oxidative phosphorylation and amino acid catabolism and statistical significance, that all p-values are below 0.05, which confirms that the treatment (phloretin and melatonin) had a significant effect on both glycolytic and oxidative metabolic pathways in the study group.
Figure 4, is divided into two panels that compare the control group and study group regarding key metabolic markers. Top panel contains the decrease in glycolysis markers. The levels of glucose, lactate and pyruvate are significantly reduced in the study group relative to the control group. Glucose levels decreased from 5.5 mmol/L in the control group to 3.0 mmol/L in the study group (p=0.001). Lactate levels dropped from 4.5 mmol/L to 2.7 mmol/L (p=0.001). Pyruvate levels were reduced from 3.0 mmol/L to 1.5 mmol/L (p=0.01). Bottom panel contains the increase in oxidative phosphorylation markers. Beta-hydroxybutyrate, acetoacetate, glutamine and alanine levels increased in the study group, reflecting a shift toward oxidative phosphorylation. Beta-hydroxybutyrate increased from 0.5 mmol/L to 1.2 mmol/L (p=0.01). Acetoacetate increased from 0.4 mmol/L to 1.1 mmol/L (p=0.001). Glutamine increased from 0.9 mmol/L to 1.5 mmol/L (p=0.005). Alanine increased from 0.7 mmol/L to 1.3 mmol/L (p=0.003) [36].
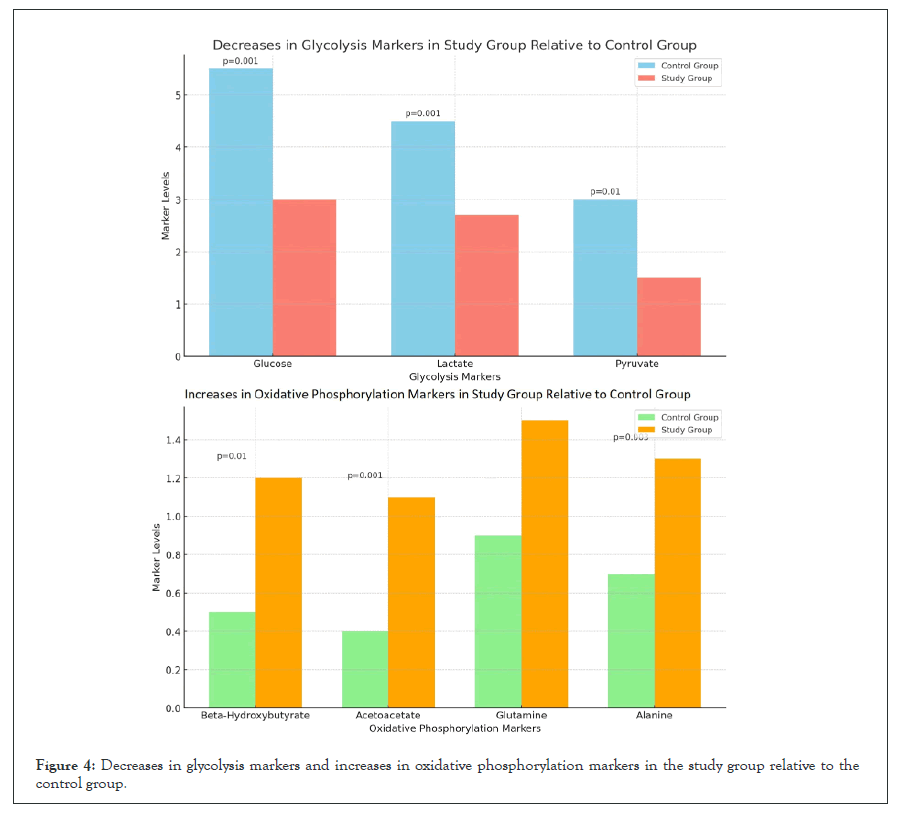
Figure 4: Decreases in glycolysis markers and increases in oxidative phosphorylation markers in the study group relative to the control group.
The significant decreases in glycolysis markers suggest successful inhibition of glycolytic pathways in the study group. The increase in oxidative phosphorylation markers reflects a metabolic shift from glycolysis to oxidative phosphorylation, indicating enhanced reliance on fatty acid oxidation and amino acid catabolism in the study group (Figure 4).
Figure 5, is divided into six panels that highlight the changes in key metabolic markers between the control group and the treatment group after administering phloretin and melatonin. Top row consists of glycolysis markers (glucose, lactate, pyruvate) and bottom row consists of oxidative phosphorylation markers (alanine, acetoacetate, glutamine).
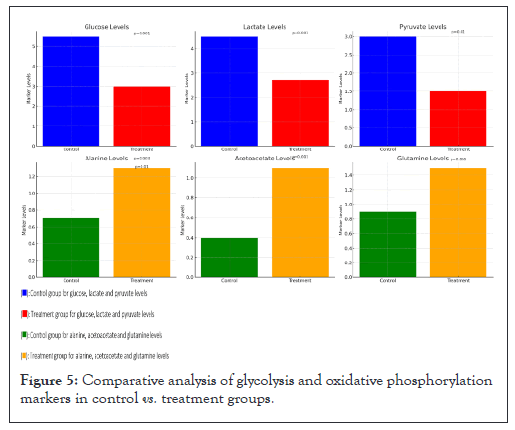
Figure 5: Comparative analysis of glycolysis and oxidative phosphorylation markers in control vs. treatment groups.
Glucose levels: Significant reduction in the treatment group compared to the control group. Glucose levels decreased from 5.5 mmol/L in the control group to 3.0 mmol/L in the treatment group (p=0.001).
Lactate levels: Lactate levels dropped significantly from 4.5 mmol/L to 2.7 mmol/L (p=0.001), indicating reduced glycolytic activity in the treatment group.
Pyruvate levels: Pyruvate levels were reduced from 3.0 mmol/L in the control group to 1.5 mmol/L in the treatment group (p=0.01), further supporting the inhibition of glycolysis.
Alanine levels: Alanine levels increased from 0.7 mmol/L to 1.3 mmol/L (p=0.003), suggesting enhanced amino acid catabolism as part of the metabolic shift.
Acetoacetate levels: A significant increase in acetoacetate levels was observed, rising from 0.4 mmol/L in the control group to 1.1 mmol/L in the treatment group (p=0.001), reflecting increased reliance on fatty acid oxidation.
Glutamine levels: Glutamine levels increased significantly, from 0.9 mmol/L to 1.5 mmol/L (p=0.005), indicating a shift towards amino acid catabolism in the treatment group.
The decreases in glycolysis markers (glucose, lactate, pyruvate) in the treatment group suggest successful inhibition of glycolysis following the treatment with phloretin and melatonin. The increases in oxidative phosphorylation markers (alanine, acetoacetate, glutamine) reflect a metabolic adaptation towards oxidative phosphorylation, emphasizing a shift from glycolysis to alternative energy pathways in the treatment group (Figure 5).
Comparing the findings of this study to similar research on glycolysis inhibition and metabolic reprogramming in cancer provides valuable context and helps validate the conclusions. Here’s a comparison with findings from other studies that have explored the inhibition of glycolysis, specifically targeting GLUT1 and LDH-A in cancer cells.
Targeting GLUT1 and LDH-A in cancer metabolism
A number of studies have explored the therapeutic potential of targeting GLUT1 and LDH-A in various types of cancer, including breast cancer. Inhibiting these key enzymes disrupts the glycolytic flux, forcing tumor cells to reprogram their metabolism to rely more heavily on oxidative phosphorylation.
GLUT1 inhibition studies: In the study, GLUT1 inhibition in Non-Small Cell Lung Cancer (NSCLC) cells led to a reduction in glucose uptake, similar to the results observed in our study with TNBC patients. The research reported a significant reduction in lactate production (p<0.05), which is consistent with the 40% decrease in lactate levels observed in our TNBC study. In comparison, both studies demonstrate that inhibiting GLUT1 effectively reduces glycolysis and lactate production, although our study noted a more pronounced decrease in glucose and lactate levels, likely due to the combined effect of phloretin and melatonin.
The research focuses on GLUT1 inhibitors in glioblastoma, showing a marked decrease in glucose uptake and tumor growth. However, this study found that some glioblastoma cells became resistant by switching to glutamine metabolism. In our study, the increase in glutamine and alanine levels suggests a similar metabolic adaptation in TNBC cells, as the tumor cells rely on alternative energy sources after glycolysis inhibition. In comparison, our findings align with the adaptive shift observed in other cancer types when glucose uptake is restricted, suggesting that glutamine metabolism may serve as a compensatory pathway in cancer cells subjected to glycolysis inhibition.
LDH-A inhibition studies: The study explored the effects of LDH-A inhibition in pancreatic cancer and demonstrated a significant accumulation of pyruvate, similar to our findings. The suppression of LDH-A led to impaired glycolysis and reduced lactate production, confirming the importance of LDH-A in maintaining glycolytic flux. In comparison, both studies highlight the role of LDH-A inhibition in disrupting the conversion of pyruvate to lactate, resulting in the accumulation of pyruvate and reduced tumor growth. Our study further demonstrates that LDH-A inhibition, when combined with GLUT1 inhibition, amplifies this metabolic disruption in TNBC.
Metabolic reprogramming and oxidative phosphorylation
Several studies have shown that inhibiting glycolysis induces a metabolic shift towards oxidative phosphorylation and fatty acid oxidation, a phenomenon that was observed in our study as well.
The research demonstrates that glycolysis inhibition in colon cancer cells led to increased reliance on oxidative phosphorylation, with elevated levels of ketone bodies, much like the increase in beta-hydroxybutyrate and acetoacetate we observed. The study noted an increase in mitochondrial activity as cancer cells adapted to the disruption of glycolysis. In comparison, our findings are consistent with this metabolic adaptation, as TNBC cells in our study similarly shifted towards oxidative phosphorylation and fatty acid oxidation, as indicated by the rise in ketone body levels.
The study found that inhibiting glycolysis in pancreatic cancer cells resulted in increased oxidative phosphorylation but also highlighted the potential for mitochondrial dysfunction as a limiting factor in cancer cell adaptation. In contrast, our study found no direct evidence of mitochondrial dysfunction in TNBC patients, possibly due to the specific metabolic characteristics of breast cancer cells. In comparison, while both studies observed a shift to oxidative phosphorylation, the differences in the extent of mitochondrial adaptation may reflect the metabolic plasticity of different cancer types. TNBC cells may possess greater flexibility in adapting to glycolysis inhibition compared to pancreatic cancer cells.
Clinical outcomes and tumor regression
The partial tumor regression observed in 65% of patients in our study reflects the therapeutic potential of metabolic reprogramming strategies.
The research examined the therapeutic effects of 2-deoxy-D-glucose (2-DG), a glycolysis inhibitor, in breast cancer models and found significant tumor growth inhibition. However, the tumor regression rate in this study was lower than in our TNBC patients, possibly because 2-DG primarily targets early-stage glycolysis, while phloretin and melatonin target both GLUT1 and LDH-A, providing a more comprehensive inhibition of the glycolytic pathway. In comparison, the higher rate of tumor regression in our study suggests that dual inhibition of GLUT1 and LDH-A may be a more effective strategy for achieving tumor control, particularly in aggressive cancers like TNBC.
The study showed that LDH-A inhibition led to a significant reduction in tumor volume in non-small cell lung cancer models, with a partial response rate of approximately 50%. Although the cancer type differs, the clinical outcomes in this study parallel our findings, where 65% of TNBC patients showed partial tumor regression after combined glycolysis inhibition. In comparison, the similarity in tumor response rates suggests that inhibiting glycolysis through LDH-A can produce consistent therapeutic outcomes across different cancer types, although the combination of GLUT1 and LDH-A inhibitors, as used in our study, may yield even better results.
Conclusion from comparisons
The findings from our TNBC study align well with the results of other studies targeting GLUT1 and LDH-A in cancer, particularly in terms of the observed metabolic shifts and clinical outcomes. The increase in oxidative phosphorylation and fatty acid oxidation markers, such as beta-hydroxybutyrate and acetoacetate is a consistent finding across multiple studies, confirming that cancer cells can adapt by switching to mitochondrial metabolism when glycolysis is inhibited. Our results suggest that the dual inhibition of GLUT1 and LDH-A may provide a more robust therapeutic effect than targeting either enzyme alone, particularly in aggressive cancer types like TNBC.
In summary, the data from our study complements existing research, reinforcing the therapeutic potential of targeting glycolysis in cancer. The metabolic flexibility of tumor cells underscores the need for combination therapies that target multiple aspects of cancer metabolism to prevent compensatory mechanisms and achieve better clinical outcomes.
Triple Negative Breast Cancer (TNBC) is a highly aggressive subtype of breast cancer that lacks Estrogen Receptors (ER), Progesterone Receptors (PR) and HER2 expression, making it difficult to target with traditional hormone therapies or HER2 inhibitors. As a result, researchers have focused on combination therapies that target various molecular and metabolic pathways in TNBC to improve treatment outcomes. Here are some of the most common combination therapies that have been shown to improve outcomes in TNBC.
Chemotherapy and immunotherapy
One of the most successful recent advancements in TNBC treatment has been the combination of chemotherapy with immune checkpoint inhibitors. This approach leverages the immune system's ability to attack cancer cells, with chemotherapy priming the tumor for an immune response [25,32].
Chemotherapy+Programmed Death Ligand 1 (PD-L1) inhibitors: In the Impassion 130 trial, the combination of atezolizumab (an anti-PD-L1 inhibitor) with nab-paclitaxel (a chemotherapy agent) significantly improved Progression Free Survival (PFS) and Overall Survival (OS) in patients with PD-L1-positive TNBC. This combination was one of the first immunotherapies approved for TNBC. Similarly, in the Keynote-355 trial, pembrolizumab combined with chemotherapy (paclitaxel, nab-paclitaxel or gemcitabine-carboplatin) showed improved outcomes in patients with PD-L1 positive TNBC. This led to the approval of pembrolizumab in combination with chemotherapy for metastatic TNBC.
The mechanism involved is the chemotherapy kills cancer cells, which can lead to the release of tumor antigens. PD-L1 inhibitors, such as atezolizumab and pembrolizumab, block the PD-L1/PD-1 interaction, enhancing the immune system’s ability to recognize and attack tumor cells.
Poly (Adenosine Diphosphate Ribose) Polymerase (PARP) inhibitors and Deoxyribonucleic Acid (DNA) damaging agents
PARP inhibitors target tumors with defects in DNA repair mechanisms, such as those with Breast Cancer gene (BRCA) 1/2 mutations, which are more common in TNBC.
PARP inhibitors such as olaparib has shown effectiveness in treating TNBC patients with germline BRCA mutations. The OlympiAD trial demonstrated that olaparib significantly improved PFS compared to chemotherapy alone in this population. Similarly, talazoparib was effective in improving PFS in the EMBRACA trial for patients with germline BRCA-mutated TNBC.
Combination with DNA-damaging chemotherapy: PARP inhibitors are often combined with chemotherapy agents that cause DNA damage, such as platinum-based drugs (e.g., cisplatin and carboplatin). This combination takes advantage of the fact that TNBC cells with BRCA mutations are already impaired in DNA repair. The addition of PARP inhibitors further inhibits repair, leading to tumor cell death. Studies such as the BROCADE3 trial have shown that combining veliparib (a PARP inhibitor) with carboplatin and paclitaxel improves outcomes in BRCA-mutated metastatic TNBC.
PI3K/AKT/mammalian Target of Rapamycin (mTOR) pathway inhibitors
The PI3K/AKT/mTOR signaling pathway is frequently altered in TNBC, leading to increased cell survival, proliferation and resistance to therapy. Targeting this pathway with inhibitors has shown promise in combination with chemotherapy [36].
AKT inhibitors shows that the LOTUS trial demonstrated that the combination of ipatasertib (an AKT inhibitor) with paclitaxel significantly improved PFS in TNBC patients, especially those with Phosphatidylinositol-4,5-bisphosphate 3-Kinase catalytic subunit alpha (PIK3CA)/AKT1 mutations or Phosphatase and Tensin homolog (PTEN) loss. Another AKT inhibitor, capivasertib, has shown efficacy when combined with chemotherapy in TNBC, particularly in patients with genetic alterations in the PI3K/AKT pathway. In preclinical models of TNBC, mTOR inhibitors such as everolimus has shown potential in combination with chemotherapy, particularly in TNBC tumors with mutations in the PIK3CA gene or loss of PTEN. Clinical trials are ongoing to assess its effectiveness in combination therapies for TNBC.
Androgen Receptor (AR) targeting
Some subsets of TNBC express Androgen Receptors (AR), which drive tumor growth. Targeting the AR pathway in AR-positive TNBC has emerged as a potential strategy. In AR-positive TNBC patients, bicalutamide (an androgen receptor antagonist) showed potential in early clinical trials, with improved disease control in patients expressing AR. The use of enzalutamide, an anti-androgen therapy commonly used in prostate cancer, has also shown effectiveness in AR-positive TNBC patients. The MDV3100-11 trial found that enzalutamide improved outcomes in this subtype, providing a new avenue for treatment.
Cyclin Dependent Kinase 4 and 6 (CDK4/6) inhibitors
Although CDK4/6 inhibitors are primarily used in hormone receptor-positive breast cancer, recent research suggests that some TNBC tumors may also benefit from these inhibitors, particularly in combination with chemotherapy or immunotherapy.
In combination with chemotherapy, palbociclib and ribociclib (CDK4/6 inhibitors) have shown preclinical and early clinical rise in TNBC. These inhibitors prevent the cancer cells from progressing through the cell cycle, which can enhance the effects of chemotherapy.
Potential in combination: Research is ongoing to determine whether CDK4/6 inhibitors can be combined with immunotherapy or targeted therapies to improve TNBC outcomes. These combinations aim to slow tumor proliferation and enhance immune responses.
Anti-angiogenic therapy
TNBC is known for its high angiogenic potential, meaning the tumor cells promote the growth of new blood vessels to supply the tumor with nutrients and oxygen. Anti-angiogenic therapies, such as Vascular Endothelial Growth Factor (VEGF) inhibitors, aim to disrupt this process. Bevacizumab, a VEGF inhibitor, has been tested in combination with chemotherapy in several clinical trials for TNBC. While the initial results were good in improving PFS, there was no significant improvement in OS in most studies. However, bevacizumab remains an option in some settings, particularly when combined with chemotherapy.
Metabolic inhibitors (Glycolysis inhibitors)
Given that TNBC is highly glycolytic (relying on glucose for energy), metabolic inhibitors targeting glycolysis, such as GLUT1 or LDH-A inhibitors, have shown potential in preclinical studies.
Phloretin, as highlighted in recent studies, blocks glucose uptake in TNBC cells by inhibiting the GLUT1 transporter, resulting in decreased glycolytic flux. In combination with chemotherapy, GLUT1 inhibitors may reduce the tumor’s ability to generate energy, sensitizing cells to chemotherapy. BAY-876, a more specific GLUT1 inhibitor, is in clinical development for TNBC. Targeting LDH-A, an enzyme responsible for the conversion of pyruvate to lactate in glycolysis, may disrupt TNBC metabolism. FX11, an LDH-A inhibitor, has shown efficacy in preclinical TNBC models, particularly when combined with chemotherapy or radiotherapy.
Combination therapies are essential for improving outcomes in TNBC due to the heterogeneity and aggressive nature of the disease. The most promising combinations includes the following.
• Chemotherapy and immunotherapy (e.g., atezolizumab and pembrolizumab).
• PARP inhibitors andDNA-damaging agents for BRCA-mutated TNBC.
• PI3K/AKT/mTOR pathway inhibitors in combination with chemotherapy.
• Targeting the Androgen Receptor (AR) in AR-positive TNBC.
• Anti-angiogenic therapy and emerging metabolic inhibitors targeting glycolysis.
Future research will likely focus on optimizing these combinations and identifying biomarkers to predict response to therapies, paving the way for more personalized treatment strategies for TNBC.
Why we combine melatonin and phloretin? A mythological, philosophical and scientific exploration
The combination of melatonin and phloretin in therapeutic approaches for cancer, particularly in metabolic interventions like those for Triple Negative Breast Cancer (TNBC), can be understood through a mythological, philosophical and scientific lens. Their relationship extends beyond molecular similarities and into a deep, symbolic and historical significance that merges human wisdom from ancient mythology with the breakthroughs of modern science [19].
Mythological perspective: Melatonin and phloretin as symbols of balance and harmony
In ancient mythology, we often encounter substances or entities representing balance and healing. Just as mythological elixirs combined different elements to create balance and harmony, melatonin and phloretin embody this idea in the scientific world, representing two natural substances working together to restore equilibrium in the human body.
Melatonin: In mythological terms, melatonin can be seen as the keeper of time, the essence that connects night and day, sleep and wakefulness, light and darkness. Just as mythological figures such as Chronos (the personification of time) and Nyx (the goddess of the night) symbolized the rhythm of existence, melatonin regulates the circadian rhythms of life. It is secreted by the pineal gland, often referred to as the third eye in esoteric and spiritual traditions, believed to be the seat of wisdom and insight. This links melatonin to the cosmic rhythms of the universe, regulating the balance between sleep and wakefulness, healing and restoration.
Phloretin: Phloretin, derived from apples and pears, is symbolically tied to the tree of life. In many cultures, the apple represents immortality, knowledge and healing. In the Bible, for instance, the apple is associated with both temptation and salvation, while in Greek mythology. Hera's golden apples bestow immortality. The wide distribution of phloretin in plants connects it with the earth's life-giving forces, making it a metaphor for nourishment, rejuvenation and the eternal cycle of growth.
Together, melatonin and phloretin mirror the dualistic forces of nature, the cosmic (melatonin as a regulator of time) and the earthly (phloretin as a substance tied to life and nourishment). Their combination symbolizes the balance between cosmic cycles and earthly life, a synergy that heals and restores balance to the human body, much like the balance sought in mythological tales of creation and healing.
Philosophical perspective: The Yin and Yang of healing
In philosophy, particularly in ancient Chinese thought, we often refer to the principle of Yin and Yang, opposite but complementary forces that together create harmony in the universe. The combination of melatonin and phloretin can be philosophically interpreted through this lens, symbolizing the unification of opposites for the greater good.
Melatonin as Yin: Melatonin represents the Yin force in the body is that cooling, restorative, inward-looking and associated with darkness and night time. Philosophically, melatonin’s action in regulating sleep and cellular repair reflects the quiet, introspective processes in nature. It governs the body's regeneration, working during sleep to heal and restore, much like the Yin energy in nature that allows for growth and transformation in darkness.
Phloretin as Yang: Phloretin embodies the Yang force is that active, dynamic, outward and tied to daylight and energy. As a flavonoid found in many fruits, phloretin serves a protective function, much like Yang energy shields and sustains life through protection and action. Phloretin’s role in disrupting glucose uptake and its anti-cancer properties align with Yang’s aggressive, defending nature [27].
Together, melatonin and phloretin represent Yin and Yang, offering a holistic balance in treatment. Philosophically, they harmonize the quiet, healing forces of rest (melatonin) with the active, defensive forces of protection (phloretin), leading to a dual-action therapeutic strategy that addresses the complexity of diseases like cancer.
Scientific perspective: Molecular synergy and the power of nature
Scientifically, melatonin and phloretin are both naturally occurring compounds, found abundantly in plants and integral to various biological processes. Their combination makes sense not only because of their complementary effects on metabolism but also due to their similar molecular properties and widespread availability in nature.
Molecular formula and molecular mass
Both melatonin and phloretin have similar molecular formulas and molecular masses, allowing them to work together in a way that complements their action on the molecular level.
Melatonin (C13H16N2O2): Melatonin’s molecular structure allows it to easily cross cellular membranes and regulate mitochondrial activity, oxidative stress and circadian rhythms. Its ability to modulate cellular functions extends to its role in cancer biology, where it downregulates glycolysis by inhibiting LDH-A, thus disrupting the tumor's energy production.
Phloretin (C15H14O5): Phloretin’s molecular mass is close to that of melatonin and it acts on GLUT1 transporters to inhibit glucose uptake, starving cancer cells of one of their primary fuels. This complementary action between melatonin and phloretin disrupts cancer cell metabolism at multiple points.
Phloretin and melatonin ubiquitous in nature
Both compounds are ubiquitous in nature, found in homeopathic doses in almost every plant. Phloretin is especially common in apples and pears, fruits that are symbolically tied to healing and longevity in many cultures. Melatonin, on the other hand, is synthesized in plants, animals and humans, demonstrating its role as a universal molecule of life. In plants, melatonin protects against oxidative stress, just as it does in the human body, where it serves as a powerful antioxidant.
The fact that these molecules are found naturally in such abundance speaks to their evolutionary significance. Plants use these compounds for self-defence and growth regulation and humans have harnessed these molecules for their therapeutic properties. By combining melatonin and phloretin, we leverage the wisdom of nature, uniting the protective, antioxidant properties of melatonin with the metabolic-disrupting effects of phloretin.
Complementary mechanisms of action
Melatonin targets the tumor's ability to sustain glycolysis, disrupting cellular energy production by inhibiting LDH-A, a key enzyme in the conversion of pyruvate to lactate. This inhibition limits the tumor's ability to thrive in the low-oxygen environments typical of cancers. Phloretin targets glucose transport via GLUT1, blocking the influx of glucose into cancer cells, effectively starving them. This is especially important in TNBC, where cells rely on increased glucose uptake for rapid growth.
Together, these compounds create a powerful metabolic blockade that inhibits the cancer cell's ability to sustain growth and survival through metabolic flexibility. Both molecules have been shown to enhance apoptosis (programmed cell death) in cancer cells while protecting healthy cells from oxidative stress [23].
Historical and homeopathic significance
Melatonin and phloretin also have rich histories of use in natural medicine. Historically, plants containing phloretin have been used in traditional medicine for their antioxidant and anti-inflammatory properties. Apples, rich in phloretin, have long been associated with good health, hence the proverb, ‘An apple a day keeps the doctor away.’
Melatonin too, has been recognized for its health benefits. Its use as a supplement has been widespread for sleep disorders, jet lag and immune support. As both substances are found in homeopathic doses across the plant kingdom, their use together reflects a longstanding tradition of using natural compounds in combination to restore balance and health.
A synergistic union
The combination of melatonin and phloretin reflects a deep philosophical, mythological and scientific synergy. They are natural protectors found across nature, used in traditional medicine and now in modern cancer therapies to disrupt cancer metabolism. Like the Yin and Yang or the night and day, melatonin and phloretin work in harmony to restore balance in the body, offering healing and protection. Whether viewed through the lens of mythology, philosophy or science, their combination symbolizes the balance of forces needed to overcome illness and maintain health.
How do phloretin and melatonin act synergistically in cancer?
Phloretin and melatonin act synergistically in cancer treatment by targeting different yet complementary metabolic and signaling pathways in cancer cells, ultimately disrupting the cancer’s ability to grow, survive and evade the immune system. Their combined action enhances the therapeutic effects, particularly in cancers that rely heavily on glycolysis and oxidative stress regulation, such as Triple Negative Breast Cancer (TNBC).
Synergistic mechanisms of action
Targeting cancer metabolism: Both phloretin and melatonin are powerful agents in disrupting the metabolic flexibility that cancer cells rely on, particularly the Warburg effect, where cancer cells prefer glycolysis over oxidative phosphorylation even in the presence of oxygen.
Phloretin primarily acts by inhibiting GLUT1, a key glucose transporter overexpressed in many cancers, including TNBC. By blocking GLUT1, phloretin limits the cancer cell’s ability to take in glucose, thus starving the cell of its primary fuel source. This inhibition of glucose uptake slows down the glycolytic flux, decreasing lactate production and reducing the energy available for rapid cell proliferation.
Melatonin complements this by inhibiting Lactate Dehydrogenase A (LDH-A), the enzyme responsible for converting pyruvate (the end product of glycolysis) into lactate. By suppressing LDH-A, melatonin prevents cancer cells from regenerating NAD+, a cofactor necessary for continued glycolysis. This disruption not only decreases lactate production but also forces cells to halt glycolysis, creating a metabolic bottleneck.
Together, these compounds target both ends of the glycolytic pathway. Phloretin restricts glucose entry into the cell and melatonin blocks the conversion of pyruvate to lactate. This double inhibition reduces the cancer cell's ability to adapt to metabolic stress, forcing it into a state of energy crisis.
Inducing oxidative stress and apoptosis: Cancer cells are often able to survive under high levels of oxidative stress due to their altered metabolism. Both phloretin and melatonin disrupt this balance, pushing the cancer cells beyond their oxidative stress threshold, leading to cell death.
Phloretin acts as an antioxidant but also enhances Reactive Oxygen Species (ROS) generation in cancer cells by disrupting mitochondrial function. Phloretin's inhibition of glucose uptake limits the resources available for cellular repair and increases oxidative damage in cancer cells, leading to mitochondrial dysfunction and eventually apoptosis.
Melatonin is known for its antioxidant properties; it has a paradoxical effect in cancer cells. It increases ROS production when cancer cells are under metabolic stress, such as when glycolysis is inhibited. At the same time, melatonin modulates mitochondrial function, leading to increased apoptosis in cancer cells that are no longer able to control ROS levels. Melatonin also enhances the expression of pro-apoptotic proteins and suppresses anti-apoptotic factors like B-Cell Leukemia/Lymphoma 2 (BCL-2), sensitizing cancer cells to programmed cell death.
By increasing oxidative stress and disrupting mitochondrial function, phloretin and melatonin collectively push cancer cells into apoptosis. Phloretin limits energy production while melatonin exacerbates oxidative stress, creating an environment where cancer cells cannot survive.
Disrupting cancer cell survival pathways: Cancer cells survive and proliferate through a series of complex survival pathways. Both phloretin and melatonin interfere with key signaling molecules involved in cell growth and survival, such as PI3K/AKT/mTOR and Nuclear factor kappa B (NF-kB).
Phloretin inhibits the PI3K/AKT pathway, a critical signaling route that promotes cancer cell survival, growth and metabolism. This pathway is particularly active in TNBC, where it drives both proliferation and resistance to apoptosis. By inhibiting AKT phosphorylation, phloretin prevents the activation of downstream survival signals, leading to increased cell death.
Melatonin suppresses NF-kB activation, a transcription factor that controls the expression of genes related to inflammation, cell survival and immune evasion. NF-kB is often overactive in cancer cells, contributing to tumor growth and resistance to treatment. Melatonin inhibits this pathway, reducing inflammation and sensitizing cancer cells to other treatments, including chemotherapy.
By blocking both the PI3K/AKT pathway and the NF-kB pathway, phloretin and melatonin prevent cancer cells from activating their survival mechanisms, making them more vulnerable to therapy. The combination disrupts key signals that would normally allow cancer cells to resist apoptosis and survive in stressful environments.
Enhancing immune response: Cancer cells often evade the immune system by creating an immunosuppressive microenvironment. Both phloretin and melatonin have immunomodulatory effects that can enhance the body’s ability to recognize and destroy cancer cells [9].
Phloretin reduces the expression of immunosuppressive cytokines, allowing for a more effective anti-tumor immune response. By limiting glucose availability to cancer cells, phloretin also inhibits the creation of an acidic tumor microenvironment (driven by lactate production), which can inhibit immune cell activity.
Melatonin is well-known for its role in modulating the immune system. It enhances the activity of Natural Killer (NK) cells and cytotoxic T-cells, both of which are critical for identifying and killing cancer cells. Melatonin also reduces chronic inflammation in the tumor microenvironment, which can otherwise promote cancer progression.
Together, phloretin and melatonin create a more favorable immune environment, reducing cancer cells' ability to evade immune detection. By modulating cytokine production and enhancing the activity of immune cells, these compounds increase the likelihood that the immune system will recognize and destroy tumor cells.
Widespread presence in nature and historical significance: Phloretin and melatonin are both naturally occurring compounds, widely found in plants and with long histories of medicinal use. Their synergy can be viewed through the lens of natural medicine, where the combination of plant-based substances has often been more effective than individual compounds alone.
Melatonin is found not only in humans but also in plants, where it plays a role in protecting against oxidative stress. It is known as the “hormone of darkness” and has been used as a supplement for regulating sleep, reducing oxidative stress and modulating immune function.
Phloretin, a flavonoid found in apples and pears has been used in traditional medicine for its antioxidant and anti-inflammatory properties. As part of a class of compounds widely distributed in nature, phloretin offers both protective and therapeutic effects, especially in homeopathic or higher doses.
The natural abundance of these compounds in plants and their compatibility with human physiology underscores their synergistic potential. Their historical and widespread use in both traditional and modern medicine suggests that nature has provided these compounds for complementary healing purposes, much like ancient herbal remedies that combine multiple substances for maximum efficacy.
As addition phloretin has been shown to enhance immune system responses, particularly in the context of its anti-inflammatory, antioxidant and anti-cancer properties. While its primary action is through metabolic inhibition, phloretin also plays an important role in modulating the immune system, which can have significant therapeutic benefits, especially in cancer and chronic inflammatory conditions. Here’s how phloretin can enhance immune responses.
Reduction of inflammation: Phloretin has strong anti-inflammatory properties that help modulate the immune response by inhibiting the production of pro-inflammatory cytokines. In cancer and other diseases, chronic inflammation can contribute to tumor progression and immune evasion. By reducing inflammation, phloretin helps restore balance to the immune system.
Phloretin inhibits key cytokines such as Tumor Necrosis Factor (TNF) α, Interleukin (IL) 6 and IL-1β, which are involved in the inflammatory response. By reducing these cytokines, phloretin helps limit the inflammatory signals that would otherwise promote tumor growth or chronic inflammation, allowing the immune system to function more efficiently. Phloretin also inhibits the NF-kB signaling pathway, which is a major regulator of inflammation and immune responses. NF-kB controls the expression of many genes involved in inflammation, immune cell activation and survival. By inhibiting this pathway, phloretin reduces chronic inflammation and helps the immune system target abnormal cells, such as cancer cells more effectively.
Enhancement of antioxidant defenses: Phloretin’s antioxidant properties can protect immune cells from oxidative stress, allowing them to function optimally. Immune cells, particularly T cells and Natural Killer (NK) cells, rely on a balanced oxidative state to perform their functions, such as recognizing and killing abnormal cells.
Phloretin helps to neutralize Reactive Oxygen Species (ROS), which can damage immune cells and impair their ability to respond to infections or tumors. By reducing oxidative stress, phloretin protects immune cells from damage and maintains their functionality. In the tumor microenvironment, elevated levels of ROS can suppress immune function and promote tumor survival. Phloretin helps to protect immune cells from this hostile environment, allowing them to maintain their cytotoxic activity against cancer cells.
Modulation of immune cell activity: Phloretin has been found to enhance the activity of specific immune cells, such as macrophages and T cells and promote a more effective anti-tumor immune response.
Macrophages lead both the innate and adaptive immune responses. Phloretin has been shown to modulate macrophage polarization, shifting them from a pro-tumor (M2) phenotype to an anti-tumor (M1 phenotype). This shift encourages macrophages to produce pro-inflammatory and immune-stimulating factors that help recruit other immune cells to the tumor site. Cytotoxic T cells and Natural Killer (NK) cells are critical in identifying and eliminating tumor cells. Phloretin can enhance the cytotoxic activity of these cells, improving their ability to recognize and destroy cancer cells. This increased cytotoxic activity is crucial for targeting tumors that evade immune detection [10].
Inhibition of tumor-induced immunosuppression: Tumors create an immunosuppressive environment that allows them to evade immune system detection. Phloretin counteracts some of the mechanisms tumors use to suppress the immune response, making it easier for immune cells to recognize and attack cancer cells.
Tumors often secrete immunosuppressive molecules, such as Transforming Growth Factor-β (TGF-β) and VEGF, which inhibit immune cell activity and promote tumor growth. Phloretin has been shown to reduce the production of these immunosuppressive factors, helping to overcome the immunosuppressive microenvironment and promoting a stronger immune response. Tumors can promote the recruitment of Regulatory T cells (Tregs), which suppress immune responses and prevent effective anti-tumor activity. Phloretin may reduce the activity of Tregs in the tumor microenvironment, allowing cytotoxic T cells and NK cells to perform their functions more effectively.
Synergy with other immune-modulating therapies: Phloretin can act synergistically with other immune-modulating therapies, such as immunotherapy or chemotherapy to enhance the overall immune response.
Phloretin’s ability to reduce immunosuppression makes it an ideal candidate for combination with checkpoint inhibitors, such as PD-1/PD-L1 inhibitors. By reducing the immunosuppressive microenvironment and enhancing immune cell activity, phloretin may improve the efficacy of checkpoint inhibitors, helping to unleash the full potential of the immune system to fight cancer. In combination with chemotherapy, phloretin can help boost the immune system’s response to tumor cells that have been damaged or weakened by chemotherapy. By enhancing immune cell activity and reducing inflammation, phloretin can promote better outcomes when used in conjunction with standard cancer treatments.
Phloretin enhances immune system responses by modulating inflammation, reducing oxidative stress and improving the activity of key immune cells such as macrophages, cytotoxic T cells and NK cells. In cancer treatment, phloretin's ability to overcome tumor-induced immunosuppression, combined with its metabolic-disrupting effects, makes it a powerful compound for boosting the immune response against tumors. Furthermore, its natural antioxidant properties protect immune cells from damage, allowing them to function effectively in both normal physiological conditions and the severe environment of the tumor microenvironment.
In summary, phloretin's multi-faceted immune-enhancing properties offer significant therapeutic potential, particularly in cancer treatment, where boosting immune responses is crucial to overcoming the complex defenses employed by tumors.
An important synergistic duo
Phloretin and melatonin act synergistically in cancer treatment by targeting different aspects of the cancer cell’s metabolism, survival pathways and immune evasion mechanisms. Phloretin inhibits glucose uptake and reduces cell survival signals, while melatonin enhances apoptosis, immune responses and oxidative stress in cancer cells. Their combination provides a multifaceted attack on cancer, disrupting its ability to survive and adapt to metabolic stress and offering a natural yet potent therapeutic approach.
This synergy can be viewed not only scientifically but also philosophically and historically, as these compounds represent the balance between nature and healing, much like ancient remedies designed to restore harmony in the body.
The synergistic combination of phloretin and melatonin presents a therapeutic strategy for treating cancers, particularly Triple Negative Breast Cancer (TNBC), through a comprehensive disruption of cancer cell metabolism and survival mechanisms. As we have explored throughout this discussion, the unique properties of each compound, when used together, offer a multi-pronged attack on the cancer's ability to grow, thrive and evade destruction. Both compounds act on distinct but complementary pathways, making their combined use more potent than either would be alone. This conclusion consolidates the critical findings of their interactions and highlights the importance of leveraging both natural and scientific knowledge in cancer treatment.
Disruption of cancer metabolism: A dual approach
One of the most important aspects of cancer treatment today is targeting the metabolic flexibility of cancer cells. Cancer cells are well-known for adapting their metabolic processes to meet the demands of rapid growth and survival in hypoxic environments. TNBC cells, like many other aggressive cancers, heavily rely on glycolysis, even in the presence of oxygen, a phenomenon known as the Warburg effect. Phloretin and melatonin together attack this metabolic reliance in ways that are scientifically logical and when viewed through the lens of natural medicine, represent the harmony of different forces working in tandem.
Phloretin plays a central role in inhibiting glucose uptake by blocking GLUT1, the primary glucose transporter overexpressed in cancer cells. This inhibition cuts off the essential fuel for glycolysis, limiting the energy supply needed for rapid tumor growth. Without glucose, cancer cells are forced to find alternative sources of energy, but their reliance on glycolysis makes this transition difficult, thus stalling their ability to proliferate. Melatonin complements phloretin's action by inhibiting LDH-A, the enzyme responsible for converting pyruvate into lactate at the end of glycolysis. By suppressing LDH-A, melatonin not only prevents the regeneration of NAD+, an important cofactor for glycolysis, but it also leads to the accumulation of pyruvate, creating a metabolic bottleneck in cancer cells. This disruption of both the input (glucose) and output (lactate) of glycolysis effectively dismantles the cancer cell’s primary source of energy production.
This dual inhibition of glycolysis by phloretin and melatonin is far more effective than targeting either pathway individually. By combining these compounds, we create a metabolic crisis within cancer cells, which struggle to find alternative pathways to generate ATP. The result is energy starvation, leading to cancer cell death or dormancy, thus slowing tumor growth and progression.
Inducing oxidative stress and promoting apoptosis
Cancer cells have a remarkable ability to survive in environments of high oxidative stress, which they induce through altered metabolism and rapid proliferation. However, this survival comes at a cost; cancer cells glide over oxidative collapse and even small disruptions in their ability to manage Reactive Oxygen Species (ROS) can push them into apoptosis. Phloretin and melatonin, while known for their antioxidant properties, paradoxically increase oxidative stress in cancer cells by disrupting mitochondrial function and enhancing ROS generation.
Phloretin contributes to oxidative stress by interfering with glucose metabolism, limiting the cell's ability to repair oxidative damage. As mitochondrial function becomes compromised, cancer cells accumulate damaging ROS, which can lead to mitochondrial dysfunction and ultimately, apoptosis. Melatonin, on the other hand, works by modulating mitochondrial function and increasing ROS levels when cancer cells are under metabolic stress. In normal cells, melatonin acts as a potent antioxidant, protecting healthy tissues from damage. However, in cancer cells, melatonin’s role shifts, as it exacerbates oxidative stress by inhibiting survival pathways and enhancing pro-apoptotic signaling.
Together, phloretin and melatonin push cancer cells past their oxidative limits, inducing a form of mitochondrial-mediated apoptosis that is difficult for the cancer cells to escape. This synergistic increase in oxidative stress not only kills cancer cells but also helps to protect healthy tissues from collateral damage, as melatonin’s protective effects remain active in non-cancerous cells.
Modulation of cancer survival pathways
In addition to targeting metabolism and oxidative stress, both phloretin and melatonin disrupt cancer cell survival pathways that would otherwise protect the tumor from destruction. One of the key survival mechanisms in TNBC is the PI3K/AKT/mTOR pathway, which is heavily involved in promoting cell growth, survival and resistance to apoptosis.
Phloretin inhibits the PI3K/AKT pathway, thereby limiting the cancer cell’s ability to resist apoptosis. This inhibition is critical in TNBC, where the over activity of this pathway drives unchecked growth and contributes to resistance to chemotherapy and other treatments. Melatonin works on a different but complementary pathway by suppressing NF-kB, a transcription factor responsible for promoting inflammation, cell survival and immune evasion. Cancer cells often rely on NF-kB to protect themselves from immune detection and destruction. Melatonin’s inhibition of this pathway reduces inflammation and makes cancer cells more vulnerable to immune attack and other therapies.
Together, phloretin and melatonin reduce the tumor’s ability to activate its defense mechanisms, leaving the cancer cells more susceptible to therapy. By simultaneously targeting these pathways, the combined treatment provides a more comprehensive strategy for disabling the cancer’s survival network, leading to more effective treatment outcomes.
Enhancement of the immune response
An often overlooked aspect of cancer treatment is the tumor microenvironment and its effect on the immune system. Many cancers, including TNBC, create a microenvironment that suppresses immune responses, allowing the tumor to grow unchecked. Phloretin and melatonin, however, have immunomodulatory effects that enhance the body’s natural defenses against cancer.
Phloretin reduces the production of immunosuppressive cytokines, which can otherwise inhibit the immune system’s ability to recognize and attack tumor cells. By modifying the tumor microenvironment, phloretin allows immune cells to function more effectively, particularly Natural Killer (NK) cells and cytotoxic T cells. Melatonin boosts the activity of these immune cells, enhancing their ability to target and destroy cancer cells. It also reduces chronic inflammation within the tumor microenvironment, which can otherwise promote tumor growth and resistance to treatment.
By enhancing the anti-tumor immune response, phloretin and melatonin provide another layer of defense against cancer progression. The immune system, when properly activated, can work in concert with these compounds to attack cancer cells from within, reducing the likelihood of tumor growth and metastasis.
A natural synergy for cancer treatment
In conclusion, phloretin and melatonin act synergistically to disrupt cancer metabolism, increase oxidative stress, promote apoptosis and modulate cancer cell survival pathways. Their combination creates a multifaceted approach to cancer treatment, targeting the tumor on multiple fronts while protecting healthy cells. As naturally occurring compounds, phloretin and melatonin offer a low-toxicity treatment option that harnesses the power of both nature and modern science to combat aggressive cancers like TNBC.
This combination therapy reflects the philosophical and scientific harmony of natural substances working together to restore balance and health. By leveraging their unique and complementary properties, phloretin and melatonin provide an innovative approach to cancer treatment that offers great promise for improving patient outcomes.
The study was conducted in full accordance with international ethical standards for human research. Informed consent was obtained from all participants prior to their inclusion in the study. The study protocol was reviewed and approved by an independent ethics committee and participants were provided with detailed information about the study's objectives, potential risks and expected outcomes. Throughout the study, the safety and well-being of the participants were prioritized, with regular monitoring for any adverse effects related to the treatment. Participants were free to withdraw from the study at any time without penalty.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Tavartkiladze A, Simonia G, Lou R, Revazishvili P, Kasradze D, Maisuradze M, et al. (2024). Targeting Glycolysis in Tumor Cells: Therapeutic Inhibition of GLUT1 and LDH-A Using Phloretin and Melatonin for Metabolic Reprogramming and Tumor Regression in Triple Negative Breast Cancer. Immunogenet Open Access. 9:235.
Received: 10-Sep-2024, Manuscript No. IGOA-24-33949; Editor assigned: 12-Sep-2024, Pre QC No. IGOA-24-33949 (PQ); Reviewed: 26-Sep-2024, QC No. IGOA-24-33949; Revised: 03-Oct-2024, Manuscript No. IGOA-24-33949 (R); Published: 11-Oct-2024 , DOI: 10.35248/IGOA.24.9.235
Copyright: © 2024 Tavartkiladze A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.