Journal of Chemical Engineering & Process Technology
Open Access
ISSN: 2157-7048
ISSN: 2157-7048
Research Article - (2023)Volume 14, Issue 1
With the increasing focus of the market towards the low carbon energy sources to address climate change problems while maintaining sustainable development, there is an increased demand to reduce the carbon footprint of the current processes in energy sector by either modifying the existing technology or developing the new technologies, which has led for the consideration of hydrogen as an energy carrier in transportation and various industrial applications. In this article we will discuss some of the prevalent processes that are being used industrially for the hydrogen production and what are the challenges that need to be addressed for reduction of their carbon footprint. Hydrogen classification based on the carbon footprint of its production process will be discussed in brief.
Hydrogen production; Process modification; Carbon capture
Despite the increasing interest and shift of the energy sector towards the renewables, a complete elimination of the hydrocarbon based processes is not possible in a near future because of continuous growth energy demand as shown in Figure 1. Simultaneous growth of climate change problem as shown in Figure 2, along with energy consumption has designated Hydrogen as an alternative source of energy which has lower carbon footprint than the current energy sources.
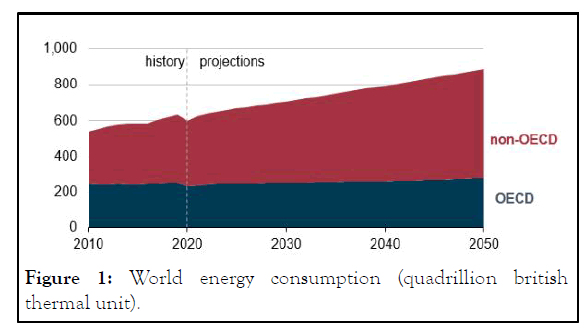
Figure 1: World energy consumption (quadrillion british thermal unit).
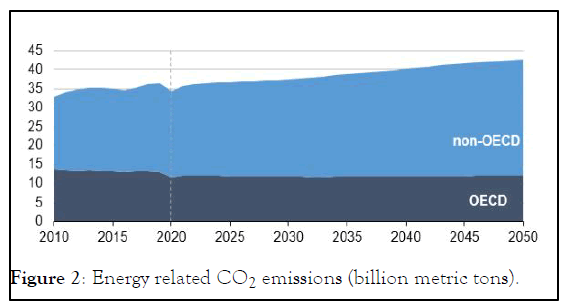
Figure 2: Energy related CO2 emissions (billion metric tons).
Currently only ~2% of globally produced hydrogen comes from renewables and the rest is from natural gas, coal, and other fossil fuel-based sources. With the increase in the hydrogen demand in future a green turnaround is technically feasible, but costcompetitiveness of green hydrogen is a pre-condition for any commercial breakthrough [1-3].
With the growing interest in the use of cleaner hydrogen (hydrogen with lesser carbon footprint) there has been an increased attraction towards the need of classification of its origin and production methods as well, given the fact that different production methods have different carbon footprint. One of the methods of classification is color classification. Based on the carbon footprints, the most common color classifications of hydrogen have been described below.
Green hydrogen: Green hydrogen is produced with no CO2 emissions. It uses clean electricity from surplus renewable energy sources, such as solar or wind power, to electrolyze water. Electrolyzers split water into its components of hydrogen and oxygen, with zero carbon dioxide emissions in the process.
Blue hydrogen: Blue hydrogen is produced from fossil fuels, mainly natural gas, by using processes like Steam Methane Reforming (SMR), Partial Oxidation (POx) and Autothermal Reforming (ATR). In these processes, carbon dioxide is produced as a byproduct which can either be captured and stored (Carbon Capture and Storage, CCS) or can be used to produce fuels chemical raw materials (Carbon Capture and Utilization, CCU).
Grey hydrogen: Grey hydrogen is created from natural gas, or methane, using steam methane reforming while the carbon dioxide produced in the process is released into the atmosphere. It is the most produced form of hydrogen in the present day power industry.
Turquoise hydrogen: This classification of hydrogen is produced by thermal decomposition of natural gas via a process called methane pyrolysis to produce hydrogen. This process produces solid carbon (soot) instead of CO2. For the CO2 neutrality of the process, it is necessary to use renewable energy to operate high temperature reactor and storage of solid carbon.
Despite the given momentum towards decarbonization, there are pertaining technological and infrastructure challenges such as transportation and storage of captured carbon dioxide, modification of existing facilities, commercialization of novel technologies etc. need to be addressed to be able to effectively move forward in the direction of carbonization.
Processes for hydrogen production
Steam Methane Reforming (SMR): Steam Methane Reforming (SMR) is a chemical process used in the gas manufacturing industry to produce hydrogen on a large scale. This process mainly contains two chemical reactions which ultimately convert water and methane (usually in the form of natural gas) into pure hydrogen and carbon dioxide. The hydrogen gas is then further purified to a quality specified by the customer. SMR is the most common and economical way to make hydrogen.
In this process natural gas is pretreated (Desulphurized) and mixed with superheated steam before it is sent to the reformer at a high temperature around and pressure where reforming reaction occurs which convert methane into carbon monoxide and hydrogen by reacting it with steam. Along with reforming reaction, water gas shift reaction also occurs in the reformer which produces further hydrogen by reacting carbon monoxide with steam. Some of the common reactions are given below.
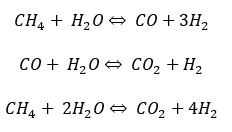
All the above mentioned reactions are highly endothermic thus external heat is required to keep the constant reactor temperature which is achieved by using natural gas as a fuel. Burning the natural gas produces carbon dioxide, which is a greenhouse gas and is one of the prime convicts of global warming. After the reformer, process gas goes to shift reactor where carbon monoxide, which is formed in the reformer gets converted to carbon dioxide and hydrogen. The last step after the shift reactor is hydrogen purification which is accomplished by pressure swing adsorption (Figure 3).
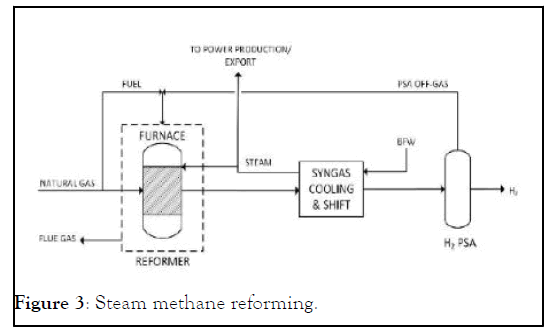
Figure 3: Steam methane reforming.
Partial Oxidation (POx): Partial Oxidation (POx), or gasification, is a chemical reaction that occurs when a mixture of a hydrocarbon feedstock and a sub-stoichiometric amount of pure Oxygen (O2) are reacted together, producing a syngas stream with a typical H2/CO ratio range of 1.6 to 1.8 [4].
The hydrocarbon feedstock is fed into the POx reactor, where the carbon in the feedstock is reacted with oxygen in an exothermic reaction, forming Carbon Monoxide (CO). Since there is a lack of oxygen, the reaction does not complete to form Carbon Dioxide (CO2).
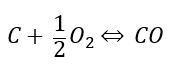
Typically, all or a portion of the CO then follows through to a water shift reaction, where the CO reacts with steam, forming a mixture of CO and H2:
There are two primary types of POX systems: 1) Thermal POX (TPOX), which occurs at >2200°F and is used with high sulfur feedstocks, and 2) Catalytic POX (CPOX), which uses low sulfur feedstocks with a sulfur-sensitive catalyst, allowing the reactions to occur in a lower temperature range of 1475-1650°F, which reduces energy consumption [5].
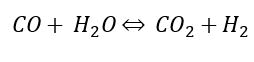
The first reaction is the reforming reaction, and the second reaction is the water gas shift reaction. Both reactions produce hydrogen and are both limited by thermodynamic equilibrium. The exit equilibrium temperature from the reformer is typically in the range of 1400°F to 1700°F. The net reaction is highly endothermic, requiring significant heat. These reactions take place under carefully controlled external firing within the reformer furnace (Figure 4).
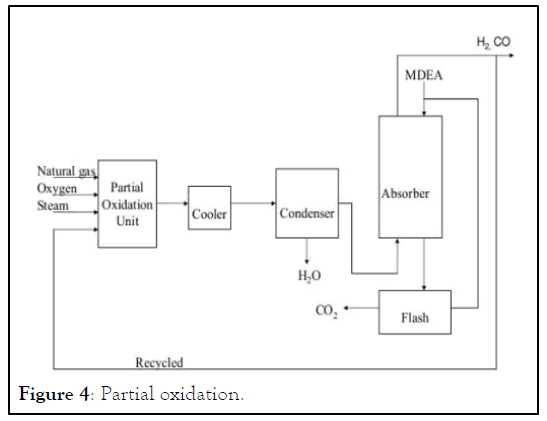
Figure 4: Partial oxidation.
Autothermal Reforming (ATR): Autothermal Reforming (ATR) uses oxygen and carbon dioxide or steam in a reaction with methane to form syngas. The reaction takes place in a single chamber where the methane is partially oxidized. The reaction is exothermic. When the ATR uses carbon dioxide, the H2:CO ratio produced is 1:1; when the ATR uses steam, the H2:CO ratio produced is 2.5:1. The outlet temperature of the syngas is between 1700°F to 2000°F. Reactions occurring in an ATR are below [6].
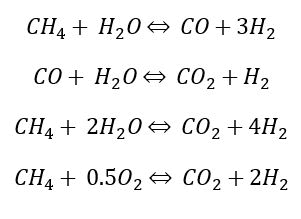
The main difference between SMR and ATR is that SMR only uses oxygen via air for combustion as a heat source to create steam, while ATR directly combusts oxygen. The advantage of ATR is that the H2:CO can be varied, this is particularly useful for producing certain second generation biofuels, such as DME (Figure 5).
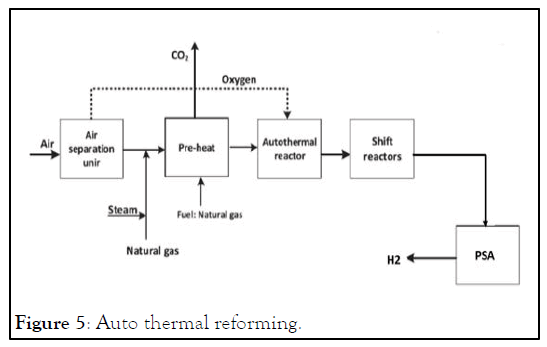
Figure 5: Auto thermal reforming.
Challenges
With the increasing focus towards DE-carbonization, there remain some challenges which still need to be overcome; some of those are described below.
Post combustion carbon capture large volumes of CO2 are produced during the combustion of natural gas. This produced CO2 is either directed towards the atmosphere or used in manufacturing industry to produce other commodities. Only a small quantity of the produced carbon dioxide is reused by manufacturing industry as well as most of the carbon dioxide eventually ends up in the atmosphere. Several methods for capturing carbon dioxide from gaseous mixtures have been designed and utilized in the industry. The purity and state of the gas in relation ambient conditions surrounding the CO2 are the factors that determine which type of technology is to be utilized. The goal of all carbon capture and storage technologies is to capture carbon dioxide from the fossil fuel based processes and store it in a geological formation. To achieve this, carbon dioxide must be compressed to a high pressure and be brought to a liquid state in order to be transported easily through pipelines and eventually pumped into a geological formation. The carbon compression stage can therefore be included as part of the CCS system.
Absorption process can be primarily divided into two stages. These are the absorption and stripping process. The absorption stage involves treated gas being in contact with solvent stream and the CO2 being captured by the solvent. The stripping involves solvent which is normally saturated with dissolved CO2 being introduced to heat to be regenerated and release the CO2 at the top of the stripping Chamber. Extent of CO2 absorption in a solvent is built around Henry’s law. Dissolution of CO2 in the liquefied solvent is due to electrostatic forces. Low temperature as well as high pressure is the best operating conditions for physical absorption. Conditions such as high temperature and low pressure are preferable for physical desorption. Physical absorbent has good absorption characteristics compared to chemical absorbent. Its regeneration can be achieved via depressurization and with a low energy demand. This is the main reason for their dominance in precombustion carbon capture technology. It must be noted that the absorption capacity of absorbent is higher at lower temperatures which implies that reducing the temperatures of treated gas streams before absorption is very important. In case of flue gases coming out of the reformer/furnace have very low pressure and high temperature, which makes it difficult to capture carbon dioxide from flue gas stream [7].
Carbon dioxide sequestration: Once the CO2 is captured, the next challenge is to find a way to utilize these large quantities of CO2. Commercial use of the CO2 would improve the economics of sequestration, but presently large scale applications are limited. Most chemical processes using CO2 require relatively small amounts, with totals in the order of millions of tons, not the billions of tons which are being produced from fossil fuel based processes. However, geological formations and the deep ocean have the potential to store the large quantities produced by fossil fuel combustion. The possible methods of sequestration are as follows.
Sequestration in geological formations
Sequestration in the deep ocean: Carbon management and sequestration has presented us with an opportunity to address climate change concerns while still enjoying the benefits of fossil fuels. However, there are several challenges that must be met.
One challenge is to reduce the cost of sequestration associated with separation and capture of CO2, verification of the feasibility of the various geologic and ocean reservoirs for CO2 storage which includes understanding the long term fate of the CO2 and addressing environmental and safety concerns. Lastly, carbon sequestration should be considered a part of an overall strategy which involves improved efficiency and non-carbon energy sources [8,9].
Cost: With coal and natural gas being readily available at a cheaper cost, the production cost of grey hydrogen can go as low as 1 $/kg H2 for regions with low gas/coal prices like the North America, Russia, and Middle East, and may stay well below 2 $/kg H2 for other regions, like Europe. While in short term cost of green hydrogen is being reported in the range of 2.5-6 $/Kg H2 meaning that green hydrogen is more expensive than grey and blue hydrogen, which is a hurdle in the way of commercializing the novel technologies. In a long term view, commercialization and innovations could contribute to cost parity with hydrogen produced from fossil fuels as shown in Figure 6. It is worth noting that the average unit size of new electrolyzers, has increased tenfold from 0.1 MWe in 2000-2009 to 1.0 MWe in 2015-2019, indicating a shift from pilot and demonstration projects in direction towards commercial-scale applications [10].
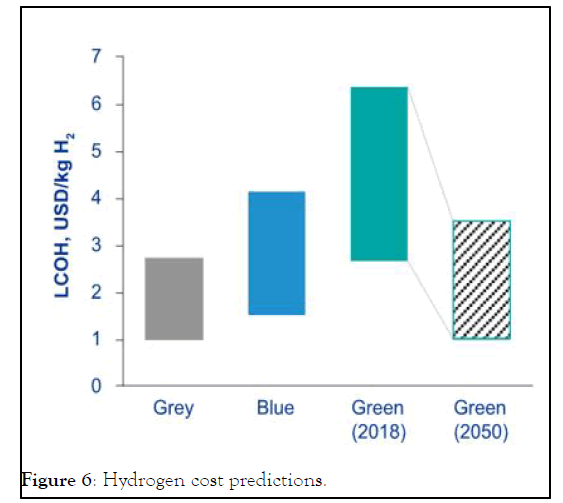
Figure 6: Hydrogen cost predictions.
Although the ongoing efforts towards carbonization, Carbon Capture and Storage (CCS) and utilization of captured carbon dioxide are increasing in number and government policies coming out in support of these efforts, we still have some hurdles to overcome to be able to effectively decarbonize the existing fossil fuel-based process, store or utilize the captured carbon dioxide and commercialize the biomass based alternative processes for energy production. Economic analysis of these processes needs to be done, as including expensive CCS technology to the already existing processes will lead to the higher prices of the produced green hydrogen as it leads to CAPEX increase for SMR.
Citation: Tripathi N (2023) Technological and Economic Challenges Faced by Green Hydrogen: An Opinion. J Chem Eng Process Technol. 14:313.
Received: 14-Sep-2022, Manuscript No. JCEPT-22-19211; Editor assigned: 16-Sep-2022, Pre QC No. JCEPT-22-19211 (PQ); Reviewed: 30-Sep-2022, QC No. JCEPT-22-19211; Revised: 17-Jan-2023, Manuscript No. JCEPT-22-19211(R); Published: 24-Jan-2023 , DOI: 10.35248/2157-7048.23.14.313
Copyright: © 2023 Tripathi N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.