Journal of Infectious Diseases & Preventive Medicine
Open Access
ISSN: 2329-8731
ISSN: 2329-8731
Research Article - (2024)Volume 12, Issue 4
Influenza is well-known to have a considerable influence on the healthcare system; therefore, vaccination plays a significant role in dealing with severe consequences. Close attention is paid to the development of tetravalent adjuvanted vaccines containing antigens of two influenzas A subtypes (H1N1, H3N2) and two influenza B subtypes (Victoria, Yamagata) to increase the effectiveness of influenza prevention. In this work, we present the results of an animal study of an innovative subunit tetravalent candidate influenza vaccine with the addition of betulin as an adjuvant, TetraFluBet. The study was conducted using female BALB/c mice, male and female mongrel rats, and guinea pigs. TetraFluBet was injected intraperitoneally twice in a 14-day interval prior to intranasal infection with 100LD50. Sub-chronic toxicity was assessed by immunohistochemistry of extracted organs and by hematological and biochemical blood parameters in guinea pigs and mongrel rats. Protectivity and immunogenicity were evaluated using the hemagglutination inhibition assay in mice. The sub-chronic toxicity study did not reveal any side effects of the candidate TetraFluBet vaccine on both rats and guinea pigs. One vaccination dose of TetraFluBet provided a pronounced degree of protective effect and significantly increased antibody titers of four influenza subtypes in response to double immunization. The candidate vaccine TetraFluBet confirmed its safety along with specific activity, showed high immunogenicity, and can be recommended for further clinical studies.
Influenza vaccine; Seasonal influenza; Pandemic influenza; Adjuvant; Subunit vaccine; Betulin
Influenza remains a substantial burden on the modern public healthcare system. According to the World Health Organization (WHO), up to 10% of the adult population and 30% of children are infected every year. Influenza, especially in risk groups, can develop into severe forms that require hospitalization and can eventually lead to death [1-4]. Meanwhile, influenza viruses retain a high pandemic potential, particularly avian influenza viruses, as well as swine and swine-origin influenza viruses [5-8]. Currently, the main approach to prevent influenza is vaccination with inactivated or subunit vaccines administered intramuscularly [9].
Prior to 2012, seasonal trivalent influenza vaccines included two strains of influenza A virus (A (H1N1), A (H3N2)) and one of the strains of influenza type B (either B1 (Yamagata-like) or B2 (Victoria-like)) [10]. It should be noted that the selected strains did not always correspond to the seasonal ones. Moreover, sometimes both strains of subtype B circulated simultaneously during the epidemic season [11]. These situations caused a significant decrease in the effectiveness of vaccination during such epidemic seasons when the vaccine strain of the influenza B virus did not correspond to the circulating strain and could not provide full protection against the wild-type virus strain line [12-13]. In such cases, the effectiveness of trivalent vaccines can be reduced by 20%-25% [14-17].
These types of vaccines may not be effective against drifted and heterologous strains because they were not included in the formula. To increase the effectiveness of influenza prevention, tetravalent vaccines were developed to target both lines of influenza B with the addition of various adjuvants [19,20].
Among the most promising adjuvants for vaccines are Nanoparticles (NPs) [21-24]. Their main advantage is that they are effectively absorbed by antigen-presenting cells [25]. As a result, an antigen bound to the NPs will be deliberately absorbed by macrophages, leading to an increased immune response. Among the spectrum of NPs, the most interesting are adjuvants based on birch bark triterpenoids, in particular NPs derived from the natural pentacyclic triterpene substance-betulin (adjuvant BET) [26-28].
In the present study, we investigated the safety and immunogenicity of a candidate tetravalent influenza vaccine containing a corpuscular adjuvant derived from natural betulin. We further examined the degree of the protective effect of TetraFluBet (TFB).
Manufacturing of subunit influenza vaccine containing corpuscular adjuvant derived from natural betulin TetraFluBet (TFB)
The technology of TetraFluBet manufacturing was described previously in the patent [1].
One dose (0.5 ml) of the TetraFluBet vaccine containing 200 μg of natural betulin-based corpuscular adjuvant, 5 μg of influenza A (H1N1) virus, 5 μg of influenza A (H3N2) virus, 5 μg of influenza B (Yamagata-like) virus, and 5 μg of influenza B (Victoria-like) virus in PBS buffer solution pH 7.3. Subtypes of influenza viruses were chosen according to the WHOrecommended composition of influenza virus vaccines for use in the 2018-2019 northern hemisphere influenza season for quadrivalent vaccines.
Animals
Female BALB/c mice (12-14 g), guinea pigs (250-340 g), male and female mongrel rats (140-220 g), originally obtained from Andreevka Animal Center (Andreevka, Russian Federation), were used in the study. Animals were fed ad libitum. All animals were housed according to Directive 2010/63/EU and the Ethical Guidelines for the Use of Animals in Research [29,30].
Sub-chronic toxicity
Sub-chronic toxicity was assessed in guinea pigs and mongrel rats. Sexually mature mongrel rats were administered 8 μl of vaccine daily for 10 days (80 μl in total), 3.2 μl of adjuvant daily for 10 days (32 μl in total), and placebo. Sexually mature guinea pigs were administered 13 μl of vaccine daily for 10 days (130 μl in total), 5.2 μl of adjuvant daily for 10 days (52 μl in total), and placebo (0.9% NaCl). The volume of injection was 0.5 ml i.m. Treated and control groups of animals were monitored for 24 days after injection.
Mortality, weight, behavior, appearance, and clinical symptoms of intoxication were registered daily. The animals of the treated and control groups were sacrificed after 11, 17, and 24 days of the experiment. Necropsy, full-body examination, and histopathological studies (Immunohistochemistry, IHC) were performed after sacrifice (Figure 1A).
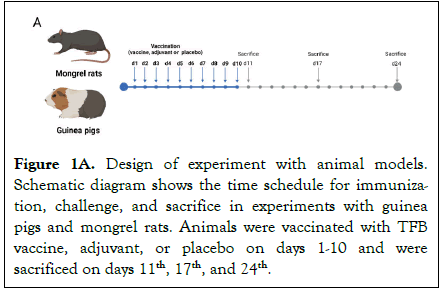
Figure 1A. Design of experiment with animal models. Schematic diagram shows the time schedule for immunization, challenge, and sacrifice in experiments with guinea pigs and mongrel rats. Animals were vaccinated with TFB vaccine, adjuvant, or placebo on days 1-10 and were sacrificed on days 11th, 17th, and 24th.
Evaluation of body weight
All animals were weighed prior to the administration of the preparation, 24 hours after the administration of the preparation, and thereafter weekly for 2 weeks.
Clinical pathology
The animals in all groups were sacrificed after 11, 17, and 24 days of the experiment (approximately once a week). At the time of sacrifice, the brain, heart, kidneys, liver, lungs, adrenal glands, thymus, spleen, lymph nodes (mesenteric and inguinal), and genitals (ovaries and testicles) of the treated and control groups were extracted and weighed. Paired organs were weighed together.
Hematoxylin and eosin staining
Extracted organs on days 11, 17, and 24 were immediately fixed in 10% neutral buffered formalin for 24 hours, followed by embedding in paraffin. Cut sections were stained with Hematoxylin and Eosin (H&E) [31].
Hematological and biochemical parameters of blood
Hematological and biochemical parameters of blood were assessed by taking blood samples from the tail vein (mongrel rats) or heart (guinea pigs) prior to injection and after 11, 17, and 24 days.
1 ml of blood was collected in test tubes with a solution of sodium citrate. The numbers of erythrocytes, leukocytes, leukocyte formula, and hemoglobin were analyzed. Blood smears were stained according to the Romanovsky-Giemsa protocol.
Biochemical parameters (glucose, total protein, creatinine, urea, bilirubin, Aspartate Amino Transferase (AST), Alanine Amino Transferase (ALT), cholesterol) were assayed using diagnostic kits (Vital Development Corporation, JSC, RF). Hematological and biochemical parameters of blood were compared using the established reference intervals.
Protectivity study
The protective effect of the TFB vaccine was assessed upon double i.p. administration of ½ human dose on days 0 and 14 in the lethal influenza model of infection of BALB/c mice with influenza subtype A/California/04/09 (H1N1) pdm09 (100 LD50) adapted to mice for intranasal administration with a volume of 50 μl.
Criteria for protectivity evaluation in mouse strains: 1) Death numbers and time of death, 2) Animal body weight
Animals were monitored for 16 days after infection. Deaths in treated and control groups were registered daily at the same time during this time period. Body weight in each group was measured daily for 5 days after the 2nd immunization and thereafter every 2 days (Figure 1B).
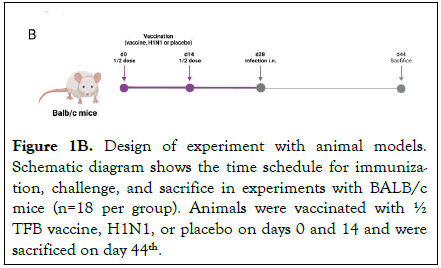
Figure 1B. Design of experiment with animal models. Schematic diagram shows the time schedule for immunization, challenge, and sacrifice in experiments with BALB/c mice (n=18 per group). Animals were vaccinated with½ TFB vaccine, H1N1, or placebo on days 0 and 14 and were sacrificed on day 44th.
Mortality (M) and Index of Protection (IP) were calculated using the obtained data of death rates (formulas No.1 and No.2).
M= (N1/N2) × 100, (1)
N1-Death rates during 16 days; N2-Total number of infected animals per group.
IP= (M1-M2)/M1 × 100, (2)
M1-Mortality rate in treated group; M2-Mortality rate in control group.
Mouse Lethal Dose (LD50) and Infection Dose (ID)
To determine one mouse lethal dose (1 LD50), 6-8-week-old female BALB/c mice (n=5) were used in a micro neutralization assay with MDCK cell culture. Animals were infected intranasally under light anesthesia with 50 μl of each dilution (5 mice per dilution). Mouse lungs were extracted on the 4th day after infection, washed in 0.01 PBS, homogenized, resuspended in 1 ml sterile 0.01 PBS, centrifuged at 2000 g for 10 min, and the obtained supernatant was analyzed. 3-3.5 × 104 cells/well were cultured in 96-well plates in Eagle's Minimal Essential Medium (MEM) containing 5% Fetal Bovine Serum (FBS), 10 mM Glutamine, and antibiotics (100 IU/ml Penicillin and 100 μg/ml Streptomycin). Confluent monolayers were washed twice with serum-free MEM before infection. 10-fold serial dilutions of the virus from whole to 10-8 for each virus with the addition of TCPK-trypsin (2 μg/ml) were prepared. 4 wells in 96-well plates with monolayers were infected with respective dilutions. Wells were rinsed 3 times after 72 h incubation (5% CO2, 37ºC) and fixed in 10% Formaldehyde (FA) (5 min RT). FA was discarded, and 100 μl of 1% crystal violet was added to each well (5 min RT). Plates were rinsed in deionized water and dried. 100 μl of 96% EtOH was added to each well and incubated at RT. OD was measured at 570 nm. Wells with OD lower by 20% in comparison to control wells were registered as positive. LD50 and ID were determined by the Reed-Muench method in 4 replicates [15].
Immunogenicity study
The study was conducted using female BALB/c mice, 18 animals per group. Animals were immunized twice i.p. with ½ TFB vaccine, H1N1, or placebo in a 2-week interval on days 0 and 14 and were sacrificed on day 44. Group 1-TFB; Group 2- H1N1 monovalent were immunized with the human dose in total (0.5 ml); Group 3 (Placebo) was administered 0.9% NaCl (0.5 ml). Immunogenicity in mice was assessed by antibody titers in the Hemagglutination Inhibition (HI) assay (n=6 per group) against subtypes A/Michigan/45/2015(H1N1), A/ Singapore/INFIMN-16-/0019/2016(H3N2), B/Colorado/06/ 2017 (B/Victoria/2/87 lineage), and B/Phuket/3073/2013 (B/Yamagata/16/88 lineage) on day 14 (1st immunization) and day 28 (2nd immunization) (Figure 1B).
Hemagglutination Inhibition (HI) assay
Determination of antibodies against influenza subtypes A/ Michigan/45/2015(H1N1), A/Singapore/INFIMN-16-/ 0019/ 2016(H3N2), B/Colorado/06/2017 (B/Victoria/2/87 lineage), and B/Phuket/3073/2013 (B/Yamagata/16/88 lineage) was performed in individual sera of treated groups in the Hemagglutination Inhibition (HI) assay. One volume of the animal serum sample was mixed with three volumes of Receptor Destroying Enzyme (RDE). The mixture was incubated at 37ºС for 18 hours, then heat-inactivated at 56ºС for 30 minutes. Sera were diluted with PBS from 1:10 to 1:1280. 50 μl of antigen influenza standard were added to each dilution. Plates were mixed gently and incubated for 1 hour at RT. Each assay included control of spontaneous agglutination of erythrocytes and control to exclude the presence of Chicken Red Blood Cells (cRBCs) hemagglutinins. A 1% (v/v) cRBC suspension was used. The readout of the assay was registered after erythrocyte sedimentation in control wells (30-40 minutes). Geometric Mean Titer (GMT) lower than <1:8 was quantified as 1:8 (the absence of signal).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (Version 5.0, La Jolla, CA, USA). Geometric Mean Titer (GMT) and mean ± SEM were calculated for quantitative data. GMT body weight comparison was performed using one-way ANOVA and the Dunn post-hoc test with adjustment for multiple comparisons of non-normally distributed continuous data between groups. Groups of data were compared between tested groups and days. Normal distribution and homogeneous variance were tested for all the variables.
Survival curves were drawn and subsequent statistical analyses were carried out using the Integrated Development Environment (IDE) RStudio (2023.09.1 Build 494 © 2009-2023 Posit Software PBC; v. 4.3.2.). The Logrank Mantel-Cox test followed by Tukey's multiple comparison test was used to assess differences in survival between groups. Throughout this article, asterisks denote significant differences at *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Sub-chronic toxicity assay
The sub-chronic toxicity study demonstrated no alterations in behavior, fur condition, or motor activity. Weight loss, decreased appetite, and mortality were not elicited during the assay upon vaccine administration. Organs of treated animals were examined macroscopically and microscopically by IHC (Figures S1 and S2).
On day 11, AST and monocyte levels were significantly higher in TetraFluBet-treated female rats. On days 17 and 24, the level of urea in the female group of rats treated with adjuvant and TetraFluBet demonstrated significant differences compared to the placebo group. Cholesterol and hemoglobin decreased by 27% Adj/74% TFB and 11% Adj/17% TFB in female rats treated with adjuvant and TetraFluBet respectively on day 17. On day 24, erythrocytes, leukocytes, hemoglobin, lymphocytes, neutrophils, eosinophils, monocytes, glucose, total protein, creatinine, ALT, and bilirubin were within physiological reference ranges and did not show any differences (Tables S1 - S3).
On day 11, the number of erythrocytes for female guinea pigs was significantly elevated by 2 times compared to the placebo (Table S2).
The administration of both vaccine and adjuvant did not elicit pathological changes, including pronounced dystrophic and necrobiotic changes in animal organs. Hematological and biochemical blood parameters (glucose, total protein, creatinine, urea, bilirubin, AST, ALT, cholesterol) of animals did not reveal significant differences before and after the injection of the vaccine and adjuvant. All values were within the physiological limits for the corresponding species (rats, guinea pigs) (Tables S1 and S2), (Figures 2A-2F).
Figure 2. Biochemical blood parameters (Urea, AST, Cholesterol) of rats and guines pigs. (A-C): Biochemical blood parameters (Urea, AST, Cholesterol) of rats on days 11, 17, and 24. (D-F): Biochemical blood parameters (Urea, AST, Cholesterol) of guinea pigs on days 11, 17, and 24. Data are depicted as Mean ± 2SD. Note: ( ): Placebo; (
): Placebo; ( ): Adjuvant; (
): Adjuvant; ( ): TetraFluBet; (
): TetraFluBet; ( ):-Male; (
):-Male; ( )Female.
)Female.
Protectivity study
The study assessed the protective effect of the TFB vaccine in a lethal influenza model using BALB/c mice infected with influenza subtype A/California/04/09 (H1N1) pdm09. The vaccine was administered twice intraperitoneally at a ½ human dose on days 0 and 14. The mice were monitored for 16 days’ post-infection with daily registration of deaths in both treated and control groups. Body weight was measured daily for 5 days after the second immunization and then every 2 days thereafter. After two consecutive immunizations, the monitoring of the clinical condition of the animals was conducted from the 28th to the 42nd day. Survival curves were plotted using death numbers and time of death data, resulting in a significantly higher survival rate in treated groups compared to the placebo group (p<0.002). The majority of deaths in the placebo group occurred between day 32 and day 38 of the study, while in the TFB group, a small number of dead animals were observed by the end of the study (from day 38 to day 42).
Maximum body weight changes and mortality of treated and control animals after infection by influenza subtype A/ California/04/09 (H1N1) pdm09 are presented. No deaths were observed in the H1N1 group of animals. Mortality in the TetraFluBet group comprised 7.7%, while almost all animals died in the placebo group by the end of the experiment (M=92%) (Figure 3).
Figure 3. Kaplan-Meier survival curve showed significantly increased mortality in the placebo group compared to the TFB and H1N1 groups (p<0.05). Black line-TetraFluBet group; red dashed line-H1N1 group; green dotted lineplacebo group. Survival analysis was performed using the log-rank (Mantel-Cox) test followed by Tukey's multiple comparison test. Results of clinical monitoring are presented starting from day 28. Note: ( )-TFB; (
)-TFB; ( )- H1N1; (
)- H1N1; ( )-Placebo.
)-Placebo.
Maximum body weight changes and mortality of treated and control animals after infection by influenza subtype A/ California/04/09 (H1N1) pdm09 are presented. The placebo group of mice lost 35.8% of body weight. Mice immunized with the TetraFluBet vaccine and the H1N1 monovalent vaccine lost 6.0% and 22% of body weight, respectively. Significant differences (Ñ?=0.002) in weight loss were observed between the control group and the TFB immunized groups upon infection by influenza subtype A/California/04/09 (H1N1) pdm09, despite the fact that the mortality in the TFB group was slightly higher than in the H1N1 group.
One vaccination dose of TetraFluBet provided 91.6% protection and showed a significant difference compared to the control group (Ñ?=0.002). The Index of Protection (IP) against 100LD50 of influenza subtype A/California/04/09 (H1N1) pdm09 for all the treated groups in one dose was 91.6%-100%. The maximum weight loss in the TFB vaccinated group was 6 and 3.6 times less compared to the placebo group and H1N1 group respectively, combined with a high degree of protective effect (Table 1).
| No. | Group | Mortality M % | IP % | Max Weight Loss % |
|---|---|---|---|---|
| 1 | TetraFluBet i.p. 1 dose | 7.7 | 91.6 | 6 |
| 2 | H1N1 i.p. | 0 | 100 | 22 |
| 3 | 0.9% NaCl (Placebo) i.p. | 92 | - | 35.8 |
Table 1. Index of Protection of TetraFluBet vaccine after treatment with a single dose upon infection by A/California/04/09 (H1N1) pdm09 in BALB/c mice.
Immunogenicity
HI assay GMTs in response to influenza subtype A/Michigan/ 45/15(H1N1) are presented in Figures 4A and 4B. On day 14 of the study, the GMT of the TetraFluBet group was 146.7 and increased 8 times to 1173.3 by day 28. Significant differences were observed between GMTs of the group immunized with one vaccine dose of TetraFluBet on day 14 and day 28 of the study and GMTs of the H1N1 immunized group (GMT day 14=106.7, GMT day 28=853.3; p<0.05) (Figures 2A and 2B). GMTs of all immunized groups were significantly higher than the control placebo group (day 14, day 28, Ñ?<0.001) (Figures 4A and 4B).
Figure 4. Geometric mean titers in the HI assay for immunogenicity of influenza subtypes A/Michigan/ 45/15(H1N1), A/Singapore/INFIMN-16-/0019/2016 (H3N2), B/Colorado/6/17, B/Phuket/3073/2013 in BALB/c mice
on day 14 after the 1st immunization. (A) and day 28 after the 2nd immunization. (B) *p<0.05, **p<0.01, ***p<0.001, Kruskal-Wallis multiple comparisons test with Dunn’s posthoc test. 
HI assay GMTs in response to influenza subtype A/Singapore/ INFIMN-16-/0019/2016 (H3N2) are presented in Figures 4A and 4B. On day 14 of the study, the GMT of the TetraFluBet group was 106.7 and increased 6.5 times to 693.3 by day 28. Significant differences were observed between GMTs of the group immunized with one vaccine dose of TetraFluBet on day 14 and day 28 of the study (Figures 2A and 2B) and placebo (day 14 Ñ?<0.01; day 28 Ñ?<0.05). GMTs of the H1N1 immunized group (GMT day 14=45, GMT day 28=16.7) were slightly higher than in the placebo group (not significant).
HI assay GMTs in response to influenza subtype B/Colorado/ 6/17 (Victoria-like) are presented in Figures 4A and 4B. On day 14 of the study, the GMT of the TetraFluBet group was 60 and increased 6.2 times to 373.3 by day 28. Significant differences were observed between GMTs of the group immunized with one vaccine dose of TetraFluBet on day 14 and day 28 of the study and placebo (day 14 Ñ?<0.01; day 28 Ñ?<0.05). GMTs of the H1N1 immunized group were at the same level as in the placebo group.
HI assay GMTs in response to influenza subtype B/Phuket/ 3073/2013 (Yamagata-like) are presented in Figures 4A and 4B. On day 14 of the study, the GMT of the TetraFluBet group was 86.7 and increased 2.6 times to 226.7 by day 28. Significant differences were observed between GMTs of the group immunized with one vaccine dose of TetraFluBet on day 14 and day 28 of the study and placebo (day 14, day 28, Ñ?<0.01) (Figures 2A and 2B). GMTs of the H1N1 immunized group were at the same level as in the placebo group.
The study was conducted on animal models to evaluate a tetravalent influenza vaccine combined with a new enhanced adjuvant made from natural betulin. The sub-chronic toxicity study did not reveal any side effects of the candidate TetraFluBet vaccine. No toxic effects on hematological and biochemical blood parameters were revealed in the sera of rats and guinea pigs. All statistically significant variations of values were within physiological reference ranges and proved the absence of pathological deviations.
The immunogenicity study of the candidate TetraFluBet vaccine in a murine vaccination model showed that titers of specific antibodies against all four influenza subtypes had a statistically significant increase after both the 1st and 2nd immunizations. GMT levels of antibodies increased 2.6-8 times from the 1st to the 2nd immunization. The H1N1 immunized group demonstrated antibody levels only against influenza subtype A/ Michigan/45/15 (H1N1) and did not show immunogenicity with regards to the other three influenza subtypes. TetraFluBet demonstrated high immunogenicity potential. Results of specific immunity assessment by the HI assay after vaccination with TetraFluBet confirmed the formation of high antibody levels against all four virus strains included in the TetraFluBet formulation. The TetraFluBet GMT response in the HI assay was dose-dependent.
One vaccination dose of the TetraFluBet vaccine provided 91.6% protection when treated with 100LD50 of A/California/ 04/09 (H1N1) pdm09 in experimental animals. We noted that the maximum weight loss in the TFB group was 16% less compared to the H1N1 group, despite the higher degree of protective effect (IP=100%), indicating lesser toxicity of the candidate TFB vaccine. The preclinical immunogenicity and protectivity study of the betulin-adjuvanted tetravalent influenza vaccine confirmed specific activity with respect to all influenza virus subtypes included in the formulation, thus it can be recommended for clinical studies applicable for commercial vaccines (single i.p. injection with vaccine containing actual seasonal influenza strains).
The animal experimental protocols used in this study were approved by the Ethics Committee of the I.I. Mechnikov Research Institute for Vaccines and Sera (protocol No. 6, April 2, 2018). Animals were maintained in accordance with the Directive of the European Parliament and of the Council 2010/63/EU dated 22 September 2010 on the protection of animals used for scientific purposes and the Sanitary Rules for the design and maintenance of experimental biological clinics in the Russian Federation (1045-73).
Conceptualization, I.K. and A.I.; methodology, investigation, A.N., M.S., E.R.-R., N.M., A.B., O.B.; writing-original draft preparation, I.T. and A.K.; writing-review and editing, I.T., M.S., and A.K.; visualization, I.T.; project administration, I.K.
30% of this research was supported by the Ministry of Science and Higher Education of the Russian Federation “Pharma-2020”.
The animal study protocol was approved by the Ethics Committee of the I.I. Mechnikov Research Institute for Vaccines and Sera (protocol No. 6, April 2, 2018).
We thank Polina Safarova for preparing the graphic data and Taras Ivanishin for administrative support.
The authors declare that they have no competing interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Tcymbarevich I, Krasheninnikova A, Sukhova M, Ivanov A, Stukova M, Romanko ER, et al. (2024) TetraFluBet: A Tetravalent Influenza Vaccine Enhanced with Natural Betulin Adjuvant. Infect Dis Preve Med. 12:371.
Received: 11-Jun-2024, Manuscript No. JADPR-24-31955; Editor assigned: 14-Jun-2024, Pre QC No. JADPR-24-31955 (PQ); Reviewed: 28-Jun-2024, QC No. JADPR-24-31955; Revised: 05-Jul-2024, Manuscript No. JADPR-24-31955 (R); Published: 12-Jul-2024 , DOI: 10.35841/2329-8731.24.12.371
Copyright: © 2024 Tcymbarevich I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : 30% of this research was supported by the Ministry of Science and Higher Education of the Russian Federation “Pharma-2020”