Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2013) Volume 2, Issue 2
Aqueous tetra-n-butyl ammonium hydroxide solution (TBAOH) is an efficient catalyst for the acylation of alcohols, phenols and thiols. This procedure is convenient, simple and suitable for the synthesis of esters and thioesters in high yields.
One of the most important priorities within organic chemistry research is to find methods and processes more compatible and more economical compared to preceding methods. To accomplish this, we use aqueous solutions and ionic liquids as solvent in large scale. It should be noted reduction of reaction’s time and the number of processes stage is to be taken into account.
Acylation of alcohols, phenols and thiols is one of very valuable and widely used transformations in organic synthesis because of their important role in the fields of biological, industry, synthetic and medicine chemistry [1-4].
In the past decades, many methods were described (reported) for the acylation of alcohols, phenols and thiols. Generally, acylation of alcohols and phenols is performed by means of acid anhydrides or acid chlorides in the presence of tertiary amines such as triethylamine, pyridine [1]. In addition to, other catalysts such as montmorillonite [5], ionic liquids [6], triflates [7-9], tributylphosphine [10], distannoxane [11], magnesium bromide [12], indium trihalides [13] and CsF–Celite have also been utilized to achieve the acylation of alcohols, phenols, thiols [14,15]. Some of the above described methods require the use of harshreaction conditions, hazardous materials, excess acylating agent, long reaction time, high temperature and low yields.However, still it is of great importance to find new useful and environmentally friendly methods with use of base catalysts for the acylation of alcohols, phenols and thiols.
Tetra-n-butyl ammonium hydroxide is a strong organic base, which also acts as a phase transfer reagent and a surfactant. It has been used as a base or additive in Aldol [16], Ullmann [17], non-Sonogashira [18] types and Knoevenagel [19] reactions, elimination [20], addition [21] reactions, as well as hydrolysis of esters and amides [22], alkylation [23], titration [24] and synthesis of nanoparticles [25] and titanium silicate [26].
In continuation of our interest in exploring new application of tetran- butyl ammonium hydroxide in organic synthetic methodologies [27-34] and attempts to develop previous methods, we wish to report acylation of alcohols, phenols under neat conditions in the presence of an aqueous solution of TBAOH at mild conditions.
In order to optimize of the reaction conditions in terms of the amount of TBAOH (20% in water), time and temperature, the reaction of benzyl alcohol (1.5 mmol) with acetic anhydride (1.0 mmol) and benzyl mercaptan (1.0 mmol) with acetic anhydride (1.2 mmol) were studied without the presence of solvent (Scheme 1) as models reactions. As can be seen from Table 1, the rate and efficiency of reactions depend on the amount of TBAOH and temperature. The best results were obtained in the presence of 2 mL of TBAOH (Table 1, entry 4) at 50°C under air (Scheme 1 and Table 1).
| Entry | Temp. (°C) | TBAOH (mL) | Thioether | Ether | ||
|---|---|---|---|---|---|---|
| Time (min) | Yield (%)* | Time (min) | Yield (%)b | |||
| 1 | 50 | 0.5 | 400 | 70 | 380 | 72 |
| 2 | 50 | 1.0 | 240 | 77 | 260 | 75 |
| 3 | 50 | 1.5 | 110 | 85 | 100 | 81 |
| 4 | 50 | 2.0 | 70 | 90 | 80 | 92 |
| 5 | 50 | 2.5 | 70 | 90 | 80 | 91 |
| 6 | 25 | 2.0 | 400 | 30 | 400 | 25 |
| 7 | 40 | 2.0 | 400 | 74 | 400 | 65 |
aModel reaction conditions: molar ratio of benzyl alcohol (benzyl mercaptan)/acetic anhydride(acetic anhydride) was 1.5 (1.0)/1.0 (1.5). The reactions run in the presence of TBAOH (20% in water) without any extra solvent under an air atmosphere conditions
bYield refer to an isolated yield by preparative chromatography
Table 1: Optimization of TBAOH amount and temperature of reactiona.
Therefore, a diverserange of dialkyl (symmetric and unsymmetric) and aryl alkyl esters and thioesters was synthesized in good to excellent yields (80-92%) in the presence of 2 mL of aqueous solution of TBAOH (Scheme 2) in optimal reaction condition. The method is very general, which aliphatic alcohols as well as phenols react easily with acyl halides andacid anhydrides was converted to corresponding esters and thioesters exclusively (Scheme 2, Table 2 and 3).
| Entry | Substrate | Reagent | Product | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| 1 | 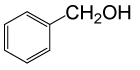 |
(CH3CO)2O | 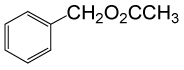 |
80 | 92 |
| 2 | 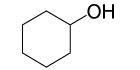 |
4- NO2PhCOCl | 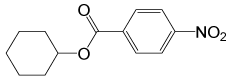 |
95 | 85 |
| 3 | 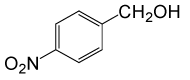 |
(CH3CO)2O | 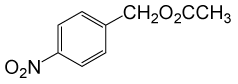 |
80 | 83 |
| 4 | 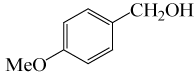 |
(CH3CO)2O | 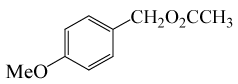 |
90 | 86 |
| 5 | (CH3)2CHOH | 4- NO2PhCOCl | 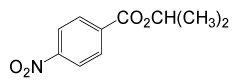 |
95 | 87 |
| 6 | 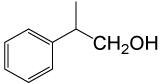 |
(CH3CO)2O | 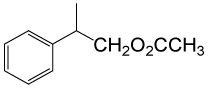 |
85 | 85 |
| 7 | 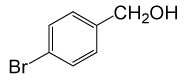 |
(CH3CO)2O | 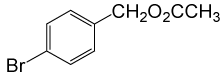 |
80 | 88 |
| 8 | (CH3)2CHOH | 4- CH3PhCOCl | 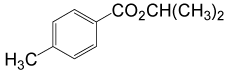 |
90 | 87 |
| 9 | 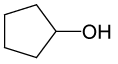 |
PhCOCl | 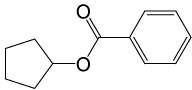 |
100 | 84 |
| 10 | (CH3)3CH2OH | PhCOCl | 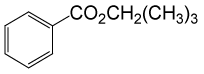 |
105 | 85 |
| 11 | 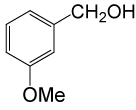 |
(CH3CO)2O | 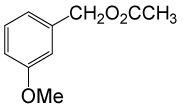 |
95 | 87 |
| 12 | CH3CH2OH | PhCOCl | PhCO2CH2CH3 | 80 | 82 |
| 13 | (CH3)4CH2OH | PhCOCl | 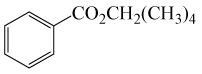 |
95 | 80 |
| 14 | PhCH2OH | PhCOCl | PhCH2O2CPh | 90 | 83 |
| 15 | 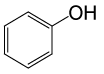 |
(CH3CO)2O | 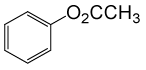 |
110 | 83 |
| 16 | 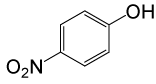 |
(CH3CO)2O | 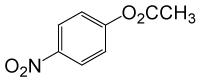 |
95 | 89 |
| 17 | 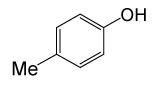 |
(CH3CO)2O | 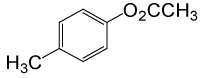 |
105 | 84 |
| 18 | 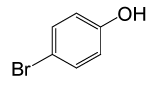 |
(CH3CO)2O | 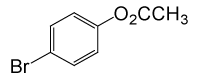 |
100 | 81 |
| 19 | 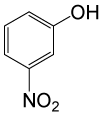 |
(CH3CO)2O | 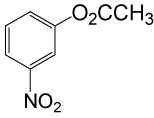 |
90 | 83 |
| 20 | 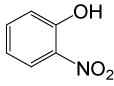 |
(CH3CO)2O | 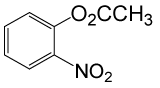 |
95 | 80 |
| 21 | 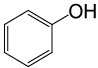 |
PhCOCl | 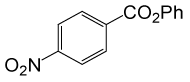 |
80 | 83 |
| 22 | 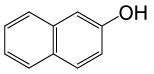 |
(CH3CO)2O | 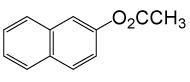 |
100 | 89 |
| 23 | 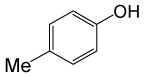 |
PhCOCl | 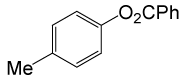 |
110 | 86 |
| 24 | 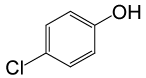 |
(CH3CO)2O | 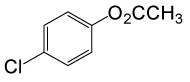 |
95 | 81 |
| 25 | 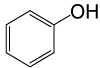 |
PhCOCl | 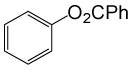 |
105 | 82 |
| 26 | 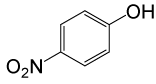 |
4-OMePhCOCl | 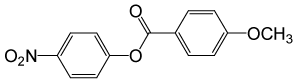 |
110 | 83 |
| 27 | 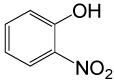 |
4-OMePhCOCl | 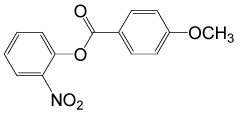 |
95 | 89 |
Table 2: Acylation of alcohols and phenols in the presence of TBAOH (20% in water)a.
| Entry | Substrate | Reagent | Product | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| 1 | 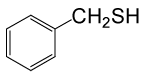 |
(CH3CO)2O | 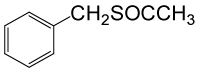 |
70 | 90 |
| 2 | 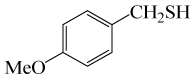 |
(CH3CO)2O | 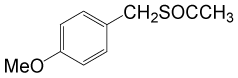 |
85 | 84 |
| 3 |  |
(CH3CO)2O |  |
80 | 86 |
| 4 | 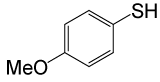 |
4-OMePhCOCl | 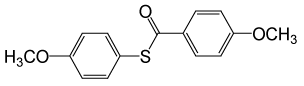 |
95 | 87 |
| 5 | 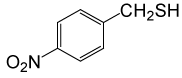 |
PhCOCl | 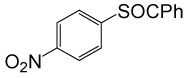 |
80 | 89 |
| 6 | 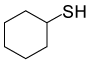 |
(CH3CO)2O | 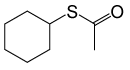 |
95 | 85 |
| 7 | 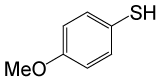 |
PhCOCl | 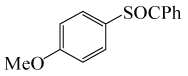 |
90 | 83 |
| 8 | 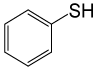 |
PhCOCl | 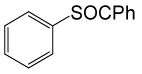 |
95 | 83 |
| 9 | 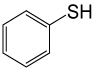 |
(CH3CO)2O | 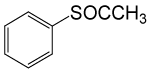 |
90 | 88 |
| 10 | 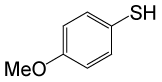 |
(CH3CO)2O | 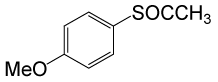 |
100 | 86 |
| 11 | 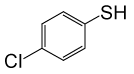 |
(CH3CO)2O | 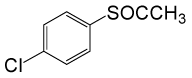 |
110 | 84 |
| 12 | (CH3)3CH2SH | PhCOCl | 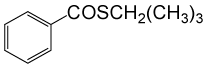 |
105 | 88 |
Table 3: Acylation of thiols in the presence of TBAOH (20% in water)a.
Products data
• Phenyl acetate (C8H8O2) (Table 2, entry 15). Yield: 83%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 2.08 (3H, s, COCH3), 7.24-7.54 (5H, m, ArH). 13C NMR (100 MHz, CDCl3): δ = 20.1, 113.8, 129.3, 130.7, 159.2, 170.5 ppm.
• Benzyl acetate (C9H10O2) (Table 2, entry 1). Yield: 92%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 2.17 (3H, s, COCH3), 5.2 (2H, s, PhCH2), 7.25-7.35 (5H, m, ArH). 13C NMR (100 MHz, CDCl3): δ = 22, 65.4, 126.9, 128.5, 128.9, 138.7, 170.4 ppm.
• 4-Bromo Phenyl acetate (C8H7BrO2) (Table 2, entry 18). Yield: 81%; colorless liquid;
1HNMR (CDCl3, 400 MHz): δ 2.45 (3H, s, COCH3), 6.9 (2H, d, J=8.8, ArH), 7.29 (2H, d, J=8.8, ArH). 13C NMR (100 MHz, CDCl3): δ = 21.1, 121.6, 125.9, 129.5, 156.8, 170.4 ppm.
• Isopropyl 4-methylbenzoate (C11H14O2) (Table 2, entry 8). Yield: 87%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 1.03 (6H, d, J=7.2, (CH3)2 CH), 2.05 (3H, s, COCH3), 4.2 (1H, m, J=7.2, ArH), 7.47 (2H, d, J=8.8, ArH).7.56 (2H, d, J=8.8, ArH). 13C NMR (100 MHz, CDCl3): δ = 20.3, 59.1, 126.8, 128.8, 132.9, 135.5, 159.1 ppm.
• 2-Naphthyl acetate (C12H10O2) (Table 2, entry 22). Yield: 89%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 2.40(s, 3H, COCH3), 7.27-7.30 (m, 1H, ArH), 7.49-7.5(m, 2H, ArH), 7.61 (d, 1H, ArH), 7.84-7.91 (m , 3H, ArH).13C NMR (100 MHz, CDCl3): δ = 21.2, 118.7, 121.3, 125.8, 126.7, 127.8, 127.9, 129.5, 131.6, 133.9, 148.5, 169.8 ppm.
IR (KBr) cm-1: 1758(C=O)
• 4-nitrobenzyl acetate (C9H9NO4) (Table 2, entry 3). Yield: 83%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 2.17(s, 3H, COCH3), 5.22(s, 2H, PhCH2), 7.53 (2H, d, J=8.8Hz, ArH), 8.23(2H, d, J=8.8Hz, ArH) ppm.
IR (KBr) cm-1:1235(C-O), 1738(C=O)
• 3-Methoxybenzyl acetate (C10H12O3) (Table 2, entry 11). Yield: 87%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 2.13 (s, 3H, COCH3), 3.83(s, 3H, OCH2), 5.11(s, 2H, PhCH2), 6.88-6.97(m, 3H, ArH), 7.30(t, 1H,ArH) ppm.
IR (KBr) cm-1:1227(C-O), 1742(C=O)
• 2-phenyl propyl acetate (C11H14O2) (Table 2, entry 6). Yield: 85%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 1.34 (d, J=6.8Hz, 3H, CH3), 2.05 (3H, J=6.8Hz, s, CH3, COCH3), 3.09-3.18(m, 1H, PhCH2), 4.14- 4.25(2H, m, J=6.8Hz, CH2CO), 7.25-7.29 (m, 3H,), 7.33-7.37 (m, 2H). 13CNMR(10MHz, DMSO)=18.1, 20.9, 38.9, 69.4, 126.7, 127.3, 128.5, 143.2, 171.1 ppm.
IR (KBr) cm-1:1233(C-O), 1741(C=O)
• phenylthioacetate (C8H8OS) (Table 3, entry 9). Yield: 88%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 3.51 (3H, s, COCH3), 7.29-7.44 (5H, m, ArH).13C NMR (100 MHz, CDCl3): δ = 29.8, 127.7, 127.8, 129.4, 137.2, 196.1 ppm.
• n-ButylThiobenzoate (C11H14OS) (Table 3, entry 12). Yield: 88%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 0.93 (3H, t, J=7.2, CH3), 1.42 (2H, sex, J=7.2, CH2CH3), 1.58 (2H, quint, J=7.2, CH2CH2), 2.45 (2H, t, J=7.2, CH2S).7.26 (1H, quint, J=7.2, ArH) 7.34 (2H, t, J=7.2, ArH).7.53 (2H, d, J=7.2, ArH). 13C NMR (100 MHz, CDCl3): δ = 13.7, 22.1, 31.1, 31.3, 127.7, 127.8, 128.5, 132.2, 137.4, 196.7 ppm.
• benzylthioacetate (C9H10OS) (Table 3, entry 1). Yield: 90%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 2.17 (3H, s, COCH3), 5.19 (2H, s, CH2S), 7.3 (1H, t, J=7.2, ArH) 7.4 (2H, t, J=7.2, ArH). 7.53 (2H, quint, J=7.2, ArH). 13C NMR (100 MHz, CDCl3): δ = 21.2, 43.8, 121.6, 127.6, 127.8, 128.5, 189.5 ppm.
• 4-methoxybenzyl thioacetate (C10H12O2S) (Table 3, entry 2). Yield: 84%; colorless liquid;
1H NMR (CDCl3, 400 MHz): δ 2.02 (3H, s, COCH3), 3.4 (2H, s, OCH3), 4.71 (2H, s, CH2S), 7.1 (2H, d, J=8, ArH), 7.4 (2H, d, J=8, ArH). 13C NMR (100 MHz, CDCl3): δ = 15.3, 55.3, 65.4, 113.8, 129.3, 130.7, 159.1, 196, 196.7 ppm.
In conclusion, we developed the utility of TBAOH as an efficient, versatile, commercially available, environmentally and economically friendly organo-basic catalyst for the preparation esters and thioesters. This method is applicable for the acylation of alcohols, phenols and thiols with acyl halides and acid anhydrides. In this way, we described a new, mild, simple and highly efficient method for the synthesis of esters and thioesters in excellent yields (80-92%) and short reaction times (70-110 min) under neat aqueous condition without using phase transfer reagent and organic solvent.
Chemicals were purchased from commercial suppliers and used without further purification. Yields refer to isolated products. Melting points were determined by an Electrothermal 9100 apparatus and are uncorrected. The IR spectra were obtained on a FT-IR Hartman- Bomen spectrophotometer as KBr disks, or neat. The 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker Avance NMR spectrometer in CDCl3 solution. The progress of the reaction was monitored by TLC using silica-gel SILG/UV 254 plates. All products are known and were characterized by comparing their physical and spectral data with those of the authentic samples.
Typical procedure: benzyl acetate synthesis (Table 2, entry 1)
A mixture of TBAOH (2.0 mL, 20% in water) and benzyl alcohol (1.5 mmol, 162 mg) was vigorously stirred at room temperature for 15 min. acetic anhydride (1.0 mmol, 102 mg) was then added to the mixture and stirring continued at 50°C for the appropriate times (Table 2) under air. The progress of reaction was monitored by TLC. After completion of the reaction, CH2Cl2 (15 mL) was added, and the mixture washed with H2O (3×10 mL). The organic layer was dried over anhydrous Na2SO4. The solvent was evaporated in vacuo to give benzyl acetate which was purified by preparative TLC (silica gel, eluent n-hexane: EtOAc = 4:1) to obtain 118 mg of the pure benzyl acetate (92%).