Journal of Cancer Science and Research
Open Access
ISSN: 2576-1447
ISSN: 2576-1447
Research Article - (2017) Volume 2, Issue 1
Background: The unusual accelerated division of group of cells is known as tumor, which may spread out through the body causing what is known as cancer. Quercetin is a well-known flavonoid which is abundant in many plants, and thought to have many therapeutic effects.
Aim of the study: The study performed to evaluate the anti-tumor potential of Quercetin against solid Ehrlich tumor in female mice.
Method: The study was carried out on 5 groups of female mice; Group I (Negative control), group II (Ehrlich tumor only), group III (Ehrlich+Methotrexate), group IV (Ehrlich+Quercetin) and group V (Ehrlich+Methotrexate +Quercetin). Estimation of Quinone Reductase and Aromatase enzymes activities, Malondialdehyde(MDA) concentration, Tumor Volume, percentage of DNA damage and Survivability were detected, in addition to genomic instability via RAPD-PCR.
Results: Under the influence of Quercetin administration to the tumor bearing mice; Quinone Reductase was increased, Aromatase was decreased, MDA was decreased, DNA damage was lowered, and Tumor volume was shrinked, RAPD-PCR showed the nearest pattern of bands to control and survivability was prolonged.
Conclusion: The results proved an anti-tumor potential of Quercetin with a suggestion of co-administration of Quercetin together with Methotrexate as the best choice for cancer treatment.
Keywords: Cancer; Quercetin; Methotrexate; DNA damage; RAPDPCR
Cancer development involves conversion of normal cells to malignant cancer cells through the generation of genetic, epigenetic and other changes, so-called ‘multi-step carcinogenesis. Each cancer shows different combinations of these changes, as revealed by wholegenome sequencing and global analysis of epigenetic regulation [1]. Solid Ehrlich tumor is an undifferentiated tumor that is frequently used in tumor studies. It is both used to develop a tumor model and in chemotherapy investigations [2].
Quercetin (3, 3’, 4’, 5, 7-pentahydroxyflavone) seems to be the most powerful flavonoid for protecting the body against reactive oxygen species, produced during the normal oxygen metabolism or induced by exogenous damage [3].
Methotrexate (MTX) (Methoblastin) is an anti-folate drug that is used in the treatment of cancer, autoimmune diseases and in induction of medical abortion. MTX competitively inhibits dihydrofolate Reductase (DHFR), an enzyme that catalyses the conversion of dihydrofolate to the active tetrahydrofolate. Folic acid is needed for DNA synthesis [4].
Quinone Reductase 1 (QR1) is an important phase II cytoprotective enzyme that converts quinones to hydroquinones, reducing oxidative cycling. It exhibits cancer protective activity mainly by inhibiting the formation of intracellular semiquinones radicals, and by generating α- tocopherol hydroquinone, which acts as a free radical scavenger. Induction of QR1 often coincides with induction of other phase II enzymes, and is therefore useful in the study of chemo preventive agents [5].
Aromatase converts androgens to aromatic estrogens through three consecutive hydroxylation reaction steps, and it is a proved target in breast cancer chemotherapy. Inhibitors of Aromatase have been shown to function as chemo preventive agents [6] Malondialdehyde (MDA) is an end-product of the radical initiated oxidative decomposition of poly-unsaturated fatty acids and, therefore, it is a frequently measured biomarker of oxidative stress [7].
Random Amplification of Polymorphic DNA (RAPD) is a type of PCR reaction, but the segments of DNA that were amplified are random. The RAPD analysis described by Williams et al. [8] is a commonly used as a molecular marker in genetic diversity studies. RAPD-PCR is one of the most reliably used techniques for detecting DNA damage as the amplification stops at the site of the damage. The changes occurring in RAPD profiles following genotoxic treatments include variation in band intensity as well as gain or loss of bands. This has been done through the analysis of band intensities and, or band gain or loss variation between exposed and non-exposed individuals. Indeed, the gain, loss or intensity differences of RAPD bands may be related to DNA damage, mutations or structural rearrangements induced by genotoxins, affecting the primer sites and or inter-priming distances [9].
Materials
A total of 100 adult female Swiss albino mice weighting (20-25 g), were obtained from the animal Farm of Vacsera, Helwan Egypt. Animals were housed under a constant temperature of 25 ± 1°C with free access to drinking water and acclimatized to laboratory conditions for one week prior to the experiment for adaptation.
The mice were divided into 5 groups, each of 20 mice, were injected as the following:
Group I (Negative control group)
Mice received intraperitoneal injection of 0.5% ethanol (the solvent of Quercetin) for 5 weeks (daily).
Group II (E)
Ehrlich Carcinoma Cells (ECC) were implanted subcutaneously (1 × 106 cells) into the right thigh of the hind limb of mice [10], then euthanized 4 weeks later at the end of the experiment.
Group III (E+M)
Ehrlich Carcinoma Cells (ECC) were implanted subcutaneously (1 × 106 cells) into the right thigh of the hind limb of mice, then Methotrexate was given by intraperitoneal injection on alternate days for 4 weeks at a dose of 2.5 mg/kg body weight starting from the next day to implantation of Ehrlich carcinoma cells [10].
Group IV (E+Q)
Quercetin was given by intraperitoneal (I.P.) injection at a dose of 50 mg/kg body weight daily for 1 week before implantation of Ehrlich carcinoma cells, then Ehrlich
Carcinoma Cells (ECC) were implanted subcutaneously (1 × 106 cells) into the right thigh of the hind limb of mice, then Quercetin I. P. Injection was continued for another 4 weeks starting from the next day to implantation of Ehrlich carcinoma cells [11].
Group V (E+M+Q)
Quercetin was given by intraperitoneal (I.P.) injection at a dose of 50 mg/kg body weight daily for 1 week before implantation of Ehrlich carcinoma cells, then Ehrlich
Carcinoma Cells (ECC) were implanted subcutaneously (1 × 106 cells) into the right thigh of the hind limb of mice, then Methotrexate (as in group III) and Quercetin (as in group IV) were given together by intraperitoneal injection starting from the next day to implantation of Ehrlich carcinoma cells.
At the end of the experiment, ten mice from each group were night fasted, then euthanized, the other ten from each group were allowed to survive until spontaneous death to determine survivability. The tumor tissue from the thigh was excised and its volume was measured using Venier Caliper, and then homogenized (in a buffer according to parameter) using tissue homogenizer for detection of Quinone Reductase, Aromatase, MDA and DNA damage. For control group which had no tumor tissue, a part of the muscular tissue in the thigh was excised and homogenized for detection of the same parameters.
Quinone Reductase was done by using an ELISA kit (Glory Science Co., Ltd, Del Rio, USA) uses Sandwich-ELISA as the method of Tumer [12]. Aromatase concentration was detected by using a doubleantibody sandwich enzyme-linked immunosorbent assay (ELISA) (Sunred Biological Technology Co., Ltd., Del Rio, USA) to estimate the level of mouse Aromatase (ARO) in samples [13].
Malondialdehyde was estimated by the method of Lahouel et al. [14]. DNA damage percentage was estimated according to Gerry et al [15]. Tumor volume based on calliper measurements was calculated by the modified ellipsoidal formula [16] and [17].
Tumor volume= 0.5 (Length × Width2)
Survivability Percentage was calculated according to the following equation [18]:

RAPD-PCR was done by using CinnaGen PCR Master Mix Kit (SinaClon BioScienceCompany, Karaj, Iran) [19].
In this study, there were 6 primers used as following:
| RAPD primers | Sequence |
|---|---|
| 1 | CTGCGCTGGA |
| 2 | TGCGCCCTTC |
| 3 | CCACAGCAGT |
| 4 | GGTCCCTGAC |
| 5 | GAAACGGGTG |
| 6 | GTGATCGCAG |
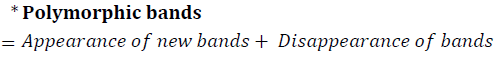

Statistical Analysis
One-way analysis of variance (ANOVA) was used to assess significant differences among groups. The Tuckey test was used to compare all groups with each other and showed the significant effect of treatment. The criterion for statistical significance was set at P<0.001, P<0.01 or P<0.05 (Graph Pad in Stat Software).
Quinone reductase (QR)
Table 1 showed that Quinone Reductase of groups II, III and V (E, E +M and E+M+Q) were significantly decreased than that of group I (Control) by (p<0.001), while group IV (E+M+Q) was decreased by (p<0.05). Although group II was elevated than group III by (p<0.001), it was decreased than group IV by (p<0.001) and group V by (p<0.01). It was clear that group III was lowered than both groups IV and V by (p<0.001). Group IV was significantly increased than group V by (<0.05).
| Group | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| Parameter | (c) | (E) | (E+M) | (E+Q) | (E+M+Q) |
| Quinone Reductase | 7.5 ± 0.2ac | 7.3 ± 0.12ade | 7.20 ± 0.15adg | 7.4 ± 0.10cdgl | 7.35 ± 0.13aegl |
| Aromatase | 133.4 ± 5.6a | 171.2 ± 5.7ad | 158.8 ± 4.6adig | 154 ± 5.8adik | 145 ± 4.5adgk |
| MDA | 63.2 ± 7.5a | 121 ± 7.9adf | 149.2 ± 6.4adg | 85.6 ± 6.4adgj | 129 ± 6.1afgj |
| DNA damage | 9.8 ± 2.3ab | 21.3 ± 3.3ade | 37.3 ± 3.8adg | 15.5 ± 3.1begj | 26.7 ± 2.7aegj |
*Values represent mean ± SD, n equals 10 for each group.
*The significance of difference was analyzed by one-way ANOVA and Tuckey test (compare all groups with each other) using computer program.
*ANOVA was significant at P<0.001, P<0.01 or P<0.05
*Tuckey test was significant at P<0.001(if a, d, g or j), P<0.01 (if b, e, h, or k)or P<0.05 (if c, f, I or l).
Table 1: Concentrations of Quinone Reductase (ng/g Protein), Aromatase (ng/g Protein), MDA (ng/g tissue) and DNA damage (%) in groups I (Control), II (Ehrlich), III (Ehrlich+Methotrexate), IV (Ehrlich+Quercetin) and V (Ehrlich+Methotrexate+Quercetin).
Aromatase
Table 1 showed that group I (control) was lower than all other groups by (p<0.001), while group II was increased than all other groups except control (group I) by (p<0.001). Group III had a higher value than group IV by (p<0.05), whilst, it was elevated than group V by (p<0.001). Although group V was higher than group I by (p<0.001), it was less than group IV by (p<0.01).
Malondialdehyde (MDA)
Table 1 showed that group I (Control) had the lowest MDA concentration between all groups, where it was decreased than groups II, III, IV and V by (p<0.001). Although Group II (E) appeared to have a less value than groups III by (p<0.001) and V by (p<0.05), it had a higher value than groups I (Control) by (p<0.001) and IV by (p<0.001) (E+Q). On the other hand, the highest value was noticed in group III (E+M) where it was elevated than all other groups by (p<0.001). Group IV was ranked as the second lowest MDA concentration next to group I (Control) and was also the closest group to control, where it was increased than control by (p<0.001), however, it was lower than groups II, III and V by (p<0.001).
DNA damage assessment
Table 1 elucidated that the lowest percentage of damage was noticed in group I (Control); where group I (control) was less than groups II, III, V by (p<0.001) and IV by (p<0.01). Group II (E) was increased than groups I by (p<0.001) and IV by (p<0.01), however, it was decreased than group III by (p<0.001) and group V by (p<0.01). It was obvious that the highest percentage of DNA damage was observed in group III (E+M), where it was elevated than all other groups by (p<0.001). Although group IV was increased than group I (control) by (p<0.01), it was lowered than groups III, V by (p<0.001) and II by (p<0.01).
Tumor volume
The results showed in Table 2 elucidated that the biggest tumor volume appeared in group II (E), where its volume was increased than all other tumor bearing groups by (p<0.001). While group III tumor was less than groups II by (p<0.001) and IV by (p<0.05), it was bigger than group V by (p<0.05).Although group IV showed a tumor volume less than group II by (p<0.001), it was increased than groups III by (p<0.05) and V by (p<0.001).
| Groups | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|
| (E) | (E+M) | (E+Q) | (E+M+Q) | |
| Tumor Volume | 1.5 ± 0.23d | 0.85 ± 0.15di | 1.1 ± 0.2dij | 0.65 ± 0.19dij |
| Survival period | 32 ± 2d | 37 ± 2dig | 40 ± 2dik | 44 ± 3dgk |
| Survivability (%) | 0d | 20 ± 3%dhg | 27 ± 4%dhj | 40 ± 6%dgj |
*Values represent mean ± SD, n equals 10 for each group.
*The significance of difference was analyzed by one-way ANOVA and Tuckey test (compare all groups with each other) using computer program.
*ANOVA was significant at P<0.001, P<0.01 or P<0.05.
*Tuckey test was significant at P<0.001(if d, g or j), P<0.01 (if e, h, k)or P<0.05 (if f, I or l).
Table 2: Tumor Volume (Cm3) and Survival period (days) in groups II (Ehrlich), III (Ehrlich+Methotrexate), IV(Ehrlich+Quercetin) and V (Ehrlich+Methotrexate+Quercetin).
Survivability
Table 2 displayed that the mice life span was prolonged in groups III, IV and V (E+M, E+Q, E+M+Q) when compared to group II (E) by (p<0.001). Although group III mice showed a prolonged life span than group II mice by (p<0.001), group III life period was shorter when compared to groups IV by (p<0.05) and V by (p<0.001). Group IV had a longer life span than groups II by (p<0.001) and III by (p<0.05), however, its life period was shorter when compared to group V by (p<0.01).
The survivability percentage was increased in groups III, IV and V (E+M+Q) than group II by (p<0.001). Although group III was increased than group II by (p<0.001), it was decreased than groups IV by (p<0.01) and V by (p<0.001). Group IV was elevated than groups II by (p<0.001) and III by (p<0.01), however, it was lowered than group V by (p<0.001) (Table 2).
RAPD–PCR
Gel electrophoresis run of RAPD – PCR product showed a polymorphic pattern of DNA bands between groups; Group II (Ehrlich), Group III (E+M), Group IV (E+Q), and Ehrlich, Group V (E +Q+M) when compared with Group I control. The results showed that the polymorphism was maximum in group II (E), then decreased gradually in groups III (E+M), IV (E+Q) and V (E+M+Q), where the lowest polymorphism was noticed in group V (E+M+Q), Table 3; Figure 1a-1f .
| Primers | Control | E | E+M | E+Q | E+M+Q | Total no. of bands | Band Size | Polymorphic bands | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | d | a | d | a | d | a | d | |||||
| 1 | 9 | 6 | 1 | 3 | 3 | 2 | 2 | - | 3 | 29 | 1200-300 | 20 |
| 2 | 8 | 5 | 2 | 6 | - | 2 | 2 | - | 2 | 27 | 1200-300 | 19 |
| 3 | 6 | 4 | 1 | 4 | - | 1 | 2 | - | 2 | 20 | 1200-300 | 14 |
| 4 | 5 | 2 | 2 | 2 | 2 | 1 | 2 | - | 2 | 18 | 1200-300 | 13 |
| 5 | 4 | 3 | - | 2 | 1 | 1 | 1 | - | 1 | 13 | 1200-300 | 9 |
| 6 | 7 | 3 | 2 | 3 | 2 | - | 3 | - | 2 | 22 | 1200-300 | 15 |
| Total | 39 | 23 | 8 | 20 | 8 | 7 | 12 | - | 12 | 129 | 90 (69.76%) | |
| a + d | 31 | 28 | 19 | 12 | ||||||||
| Polymorphism | 79.48% | 71.79% | 48.71% | 30.76% | ||||||||
a: refer to appeared polymorphic bands.
d: refer to disappeared polymorphic bands.
Table 3: Data extracted from gel electrophoresis output imaging.
Regarding the present study, group IV that treated with Quercetin only (E+Q) showed the highest value of Quin006Fne Reductase (QR), and there was a significant increase when compared with Ehrlich group (E). These results reflected the efficacy of Quercetin as antitumor agent where it caused an elevation in QR. In agreement with the present study, Jun and Rui [20] reported that Quercetin is an inducer for QR after their study of the Induction of phase II enzyme, Quinone Reductase, in murine hepatoma cells in vitro by grape extracts and selected phytochemicals including Quercetin.
Concerning Methotrexate (M), the lowest value of QR in the present study was observed in the tumor group which treated with Methotrexate only (E+M), the finding which was supported by the oxidative stress induced by Methotrexate. The recent study suggested a mechanism for correlation between Methotrexate and QR decrease depending on NADPH which necessary for QR to perform its function, and according to interpretation of Joshua and Mohamad [21], about Methotrexate chemotherapy, where they stated that Methotrexate causing a depletion of NADPH and pentose phosphate cycle enzymes during its well-known inhibitory effect on dihydrofolate Reductase. It was noted that co-administration of Quercetin with Methotrexate, as a powerful antioxidant, raised QR in group V (E+Q +M), the fact which reflected an amelioration in oxidative stress status.
Kellis and Vickery [22] had reported that Quercetin had been found to be moderately active inhibitor of Aromatase during their study on Inhibition of human estrogen synthetase (Aromatase) by flavones. In addition, Monteiro et al. [23] stated that Quercetin was one of most potent polyphenols in inhibiting Aromatase activity, during their study on modulation of Aromatase activity by diet polyphenolic compounds. In agreement with these studies, the current study confirmed the Aromatase inhibitory power of Quercetin.
Regarding Methotrexate, the present study showed a suppressing effect of Methotrexate on Aromatase.
In addition, it was noted that co-administration of Quercetin and Methotrexate in group V (E+Q+M) showed the lowest Aromatase concentration among all groups, the thing which may be due to gathering of Aromatase inhibitory effects of both (Quercetin and Methotrexate).
Sashi et al. [24] studied the potential roles of dietary agents (including Quercetin) exerting antioxidant properties that may impede cancer progression, and reported an anti-oxidative effect against oxidative stress status in cancer.
In addition, Mohamed et al. [25] reported that Quercetin was attenuating the oxidative stress during their study of the effect of Quercetin on some oxidative stress markers (including MDA), where Quercetin caused a significant decrease of MDA.
In consistence with these studies, the present study showed a significant decrease in MDA in the tumour group (E+Q) treated with Quercetin when compared with group II (Ehrlich), the matter which is confirming the previously reported anti-oxidative activity of Quercetin.
Concerning Methotrexate, Ozcicek et al. [26] reported the elevation of MDA levels under the effect of Methotrexate in rat ileum .In addition, Devrim et al. [27] declared that there was a significant increase in MDA level in Methotrexate treated group in rat kidney tissue.
In agreement with these studies, the present study confirmed the Methotrexate induced oxidative stress through the level of MDA which was the highest among all groups in tumor bearing group treated with Methotrexate only.
It is important to note that co-administration of Quercetin (as a powerful antioxidant) with Methotrexate lowered MDA in group V (E +Q+M).
Biasiak et al. [28] stated that Quercetin is ameliorating DNA damage during the study of Quercetin effect on DNA damage induced by N-methyl-Ń-nitro-N-nitrosoguanidine (MNNG). Additionally, Jin et al. [29] showed the positive effect of Quercetin against Benzo [a] pyrene - induced DNA Damages and Pulmonary Pretumorous Pathologic Changes in Mice. Also, Lin et al. [30] reported the role of Quercetin in protection against cooking oil fumes-induced DNA damage in human lung adenocarcinoma. The present study showed results which were consistent with these studies, suggesting that the antioxidative power of Quercetin may contribute to this positive effect on DNA.
In the present study, Methotrexate showed a DNA damaging action where the percentage of DNA damage was increased in tumor bearing group treated with Methotrexate (E+M) when compared with which had no treatment with tumor. In consistence with this, Vivekkumar et al. [31] reported the genotoxic effect of Methotrexate during their study of the Methotrexate-oxidative stress and genotoxicity in rat intestine. The present study suggested that DNA damage caused by Methotrexate may be oxidative stress-induced. It is worth to mention that in group V (E+Q+M) DNA damage percentage was decreased than in III (E+M), the finding which may be due to Quercetin coadministration with Methotrexate, where group IV (E+Q) showed the least DNA damage between all groups, the finding which suggested Quercetin has positive effect on DNA damage.
In agreement with the present study, Zhang et al. [32] reported that due to Quercetin treatment, the tumor volume decreased, during their study of effect of Quercetin on breeding and apoptosis of cervical cancer Hela cell and on growth of transplanted tumor in nude mice.
Concerning Methotrexate, Ahmed [33] stated that Methotrexate administration caused a decrease in the tumor volume during his study of the effect of combination between Methotrexate and histone deacetylase inhibitors on transplantable tumor. In consistence with this study, the current study showed the power of Methotrexate as chemotherapy by its ability to lower the volume of the tumor, which was more effective than Quercetin.
The present study showed a polymorphic pattern of DNA between groups through RAPD-PCR products on gel electrophoresis. This was in agreement with Sathees et al. [34] who reported a change in DNA bands in Quercetin and ellagic acid antitumor efficacies in animal models and cancer cell lines. This finding is due to an intercalation of Quercetin with DNA as a reason for the change in banding on gel.
Based on all previous results, it was logic for survivability to be increased by co-administration of Quercetin and Methotrexate together. This may be due to many reasonable facts included amelioration of oxidative stress, decreasing of Aromatase which corelated to tumor incidence and shrinkage of tumor mass. Shikha et al. [35] declared that Quercetin had the power to increase the life span of tumor bearing mice during their study to evaluate the antitumor efficacy of Quercetin, the current study showed results were consistent with that study.
The present study confirmed the anti-tumour power of Quercetin and suggested the co-administration of Quercetin and Methotrexate as an effective protocol for cancer treatment.
The study was commenced on after an approval from animal ethical and scientific committees.