Journal of Fertilization: In Vitro - IVF-Worldwide, Reproductive Medicine, Genetics & Stem Cell Biol
Open Access
ISSN: 2375-4508
+44 1478 350008
ISSN: 2375-4508
+44 1478 350008
Research Article - (2017) Volume 5, Issue 2
Objective: This study aimed to assess the accuracy of the elevation of serum uric acid in the first trimester as a predictive test for development of gestational diabetes mellitus. Patients and methods: It was a prospective observational study included 200 pregnant patients who were regularly attending the out-patient clinic for routine antenatal care to find if the elevated first trimester uric acid is associated with development of GDM or not. Results: The risk of developing GDM was higher if first-trimester uric acid was <3.1 mg/dL. Women who developed GDM were significantly older when they compared to women who did not develop GDM (Normal with Mean+SD 24.53+4.40 years, Abnormal with Mean+SD 32.78+8.18 years, p-value 0.016). It was found that, the mean BMI was significantly higher in women who developed GDM when compared to women who did not develop GDM (Normal 115 cases of total sample and no one developed GD with Mean+SD, 95.57+12.32, Overweight 59 cases, 56 cases (29.3%) were normal and 3 cases (33.3%) had GD with Mean+SD 106.29+26.62, Obese 26 cases, 20 cases (10.5%) were normal and 6 cases (66.7%) had GD with Mean+SD 124.27+39.78, p-value 0.000). Conclusion: Elevated first-trimester uric acid concentration was correlated with an increased risk of developing GDM.
<Keywords: Hyperuricemia, Gestational diabetes
Gestational diabetes mellitus (GDM) is defined as glucose intolerance that was not present or recognized prior pregnancy and it is diagnosed when the pancreatic function in women is not sufficient to control the diabetogenic environment that pregnancy confers [1]. The diagnosis of GDM also identifies pregnancies at increased risk of perinatal morbidity [2]. The incidence of GDM differs among ethnic populations, with higher rates in African Americans, Hispanics, American Indian and Asian women than in white women; values range from 1.4% to14% but overall the condition commonly affects between 2% and 5% of pregnant women [3].
The frequency of GDM varies in direct proportion to the prevalence of type II diabetes in populations, and women who develop GDM during pregnancy have a higher risk of developing type II diabetes (T2DM) later in their lives. These observations are important for relating the two pathological situations and they probably arise from common physiopathological mechanisms [4]. These are two different methods for classifying diabetes in pregnancy. The most recent is American Diabetes Association (ADA) classification [5]. Several risk factors are associated with the development of GDM. The most common is obesity (Body mass index over 30) diagnosed before pregnancy [6]. Being a member of an ethnic group with a higher rate of type II diabetes as mentioned above, polycystic ovarian syndrome [7], essential hypertension or pregnancy related hypertension [8], strong family history of diabetes in first degree relatives and a history of GDM in a previous pregnancy are other important risk factors [9]. Nevertheless, no risk factors are known in around 50% of patients with GDM.
There is enough evidence to assert that T2D has a strong genetic component. The concordance of T2D in monozygotic twins is approximately 70% compared with 20%-30% in dizygotic twins [10]. Uric acid is the main product of purine metabolism and is formed from xanthine by the action of xanthine oxidase. Normal serum uric acid levels are generally 2-7 mg/100 ml for men and 2-6 mg/100 ml for women, frequently expressed as mg%. The reason is that estrogen promotes excretion of uric acid during the productive period. The limit of uric acid solubility in extracellular fluids is 7.0 mg/dL and patients with higher serum concentrations are considered hyperuricemic [11]. Uric acid is primarily excreted via the kidney, where it is completely filtered at glomerules, fully reabsorbed into the proximal tubules, and then secreted (about 50% of the filtered load) and again reabsorbed. Elevated serum uric acid concentrations can result from an overproduction of uric acid but are usually the consequence of its low excretion. High serum uric acid levels are associated with alcohol intake, a purine-rich diet, compromised renal function and obesity. Insulin increases both sodium and uric acid reabsorption [12]. Therefore, increased serum uric acid levels may be an expression of an insulin resistant state and metabolic syndrome. This proposition is supported by evidence that higher serum uric acid levels correlate with a lower insulin response to oral glucose loading [13]. The association of high serum uric acid with insulin resistance has been known since the early part of the 20th century, nevertheless, recognition of high serum uric acid as a risk factor for diabetes has been a matter of debate. In fact, hyperuricemia has always been presumed to be a consequence of insulin 23094 resistances rather than its precursor [14]. However, it was shown in a prospective follow up study that high serum uric acid is associated with higher risk of type II diabetes independent of obesity, dyslipidemia and hypertension [15]. During pregnancy, maternal serum uric acid levels initially fall, with a subsequent rise to pre-pregnancy levels near term [16]. The third trimester rise in uric acid levels may be related to an increase in fetal uric acid production or decrease in uric acid clearance [17]. This study aimed to assess the accuracy of the elevation of serum uric acid in the first trimester as a predictive test for development of gestational diabetes mellitus.
This study was conducted at Ain Shams University Maternity Hospital from January 2016 to 31 December 2016. It was a prospective observational study which included 200 pregnant women in their first trimester who regularly attended the outpatient clinic for routine antenatal care. Serum uric acid was measured for all patients between 9-13 weeks. The patients underwent a screen test for gestational diabetes at 24-28 weeks. The aim is to determine serum uric acid could predict gestational diabetes in the 2nd trimester.
Sample size (Population): The study included 200 pregnant women during their 1st trimester and 2nd trimester. Sample size was calculated using the following equation for calculating sample size of the descriptive follow up studies, as a sample size of 162.
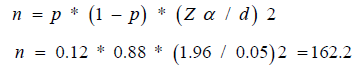
Where:
n=sample size.
p=Proportion of GDM among pregnant women with high uric acid level (from previous studies).
d=the distance (or tolerance); how close to the proportion of interest the estimate is desired to be (e.g. within 0.05).
We used a two sided Zα value for an α of 0.05
But drop out during the study is expected to be around 40 cases, so the total sample size will be 200.
Inclusion criteria
• Age of participants: Between 20-35 years old
• Pregnancy not exceeding 13weeks gestation (sure reliable dates and by using ultrasound measurement of CRL “during 1st trimester 9-10 weeks”)
• Single viable fetus
• Pregestational diabetes
• Multiple pregnancies
• Cardiovascular diseases
• Smoking
• Gout
• Renal diseases
• Liver diseases
• Thyroid diseases
• Drugs known to increase uric acid level in the blood such as aspirin, caffeine, diuretics and phenothiazines
All subjects were fully counseled for their approval to be included in this study and they all signed written informed consent.
Methods
Ethical committee approval consents of patients were taken.
Personal history: As name, age, address, consanguinity, special habits as smoking …etc.
Menstrual history: The first day of last normal menstrual period (LNMP), regularity of cycles and previous hormonal treatment.
Obstetric history: Previous deliveries, mode of deliveries, number and sex of living children, date of last labour, previous abortions, date of last abortion, puerperium and any complications like gestational diabetes, macrosomia, etc.
Past history: Of systemic or endocrine diseases as DM, hypertension, hyperthyroidism, renal diseases, etc.
Medical history: Especially of hormonal treatment and drugs known to increase uric acid level in the blood such as phenothiazines.
Family history: of diabetes mellitus, hypertension, etc.
Clinical examination
General examination: Pulse, temperature, blood pressure, body weight, body mass index and chest and heart examination, albumin, sugar and acetone in urine using dipstick.
Abdominal examination: Special palpation of the gravid uterus at the second visit between 24-28 weeks gestation by Leopolds maneuvers as fundal grip, first pelvic grip.
Ultrasonography
The ultrasound scanning was done in the fetal unit in the Obstetrics and Gynecology Department, Ain Shams University Maternity Hospital.
Technique: Ultrasound scanning in the first trimester was done using trans-abdominal approach.
Transabdominal pelvic sonography was performed with a full bladder using a transducer of 3.5 MHZ frequency.
The purpose: Obstetric ultrasound was primarily used to:
a) Date the pregnancy (gestational age). The most accurate measurement for dating is the crown-rump length of the fetus, which can be done between 9 and 13 weeks of gestation.
b) Confirm fetal viability.
c) Check the number of fetuses.
Maternal serum uric acid was measured between 9 to 13 weeks gestation.
Collection of blood samples
Venous blood sample of 3 ml was collected from each subject after placement in a dry, clean, non-contaminated glass tubes and left for half an hour to allow clotting of the blood and separation of the serum to measure uric acid and creatinine levels.
The specimens were centrifuged immediately thereafter for 5 min at 4000 round/min and the supernatant serum was transferred into another dry, clean, non-contaminated eppendrof tubes, immediately frozen and stored at -30°C until assayed.
Serum uric acid was measured in Ain Shams University Hospital main clinical laboratories chemistry department by enzymatic photometric test using a diagnostic kits (Dia Sys “Diagnostic Systems International” from Lab Top) using TBHBA (reagent 1) (2.4.6-tribromo-3-hydroxy benzoic acid) by uricase method (in uric acid) and sodium picrate (reagent 2).
Uric acid solution prepared by mixing reagent 1 and reagent 2 equally. The mixture of the two reagents still effective at temperature 3-8°C for 3 weeks and for 7 days at room temperature. 20 μ of previously prepared serum was added to 1 ml of the working reagent 1-2 and left for 5 min at temperature 37°C and for more 10 min at room temperature. The measurement instrument was set at reading 510 nm. After 15 min and changing of the color of prepared solution, it was put in the instrument and the result was obtained.
Screening for GDM
All patients underwent routine GDM screening with 50gm oral glucose-Loading test (GLT) between 24-28 weeks gestation When plasma glucose level after 1 h was <140 mg/dL, the patient was considered to be at increased risk for developing GDM and underwent 3 h oral glucose test (OGTT).
Measurement of OGTT
Preparation: The Patient was instructed not to restrict carbohydrate intake in the days before the test. The test was not done during an illness, as it may not reflect the patient’s glucose metabolism when healthy. A full adult dose was not given to a person weighing less than 43 kg or exaggerated glucoses may produce a false positive result. The OGTT was performed in the morning as glucose tolerance can exhibit a diurnal rhythm with a significant decrease in the afternoon. The patient was instructed to fast (except for water) for 8-12 h prior to the tests.
Procedure:
1. A zero time (baseline) blood sample was drawn.
2. The patient was given 100 g of glucose solution to drink within a 5 min time frame. If vomiting was repeated in another day of the test, the patient was excluded from the study.
3. Blood was drawn after 1, 2 and 3 h for measurement of glucose.
Interpretation of OGTT results: Patients were considered to have GDM if 2 or more of the 4 values exceeded the following in blood (American Diabetes Association 2009):
• Fasting blood glucose level <95 mg/dL
• 1 h blood glucose level <180 mg/dL
• 2 h blood glucose level <155 mg/dL
• 3 hour blood glucose level <140 mg/Dl
First try outcome: To see accuracy, sensitivity and specificity of elevated serum uric acid in 1st trimester to predict the development of gestational diabetes in 2nd trimester.
Second try outcome: The relationship between elevated serum uric acid in 1st trimester and the development of other gestational comorbidities in 2nd trimester.
Data were collected, revised, edited on PC and then analyzed statistically.
Statistical analysis: Analysis of date was done by SPSS for windows version”17” as follows:
• Description of quantitative variables as means, SD and range.
• Description of quantitative variables as number and percentage.
• Unpaired t-test was used to compare two groups as regard quantitative variable in parametric data (SD>50% mean).
• Mann Whitney willcoxon test was used to compare two groups as regard non parametric data (SD<50% Mean).
• ROC (Receiver operator characteristic curve was used to find out the overall productivity of parameter in and to find out the best cut of value with detection of sensitivity, specificity at this cut off value.
• Sensitivity=true+ve/true+ve+false–ve=ability of the test exclude negative cases.
• PPV (positive predictive value) =true-ve/true-ve+false– ve=ability of the test exclude negative cases.
• NPV=true-ve/true-ve+false–ve=%of the true+ve cases to all negative cases.
• Accuracy=true+ve/true+ve+true–ve
P value<0.05 insignificant
P value>0.05 significant
P value>0.01 highly significant
We started our study and 273 pregnant women were included in it. 14 pregnant women refused to continue, 25 abortions occurred during 1st trimester and 3 abortions during 2nd trimester and 31 cases were excluded during the study because they had repeated vomiting after drinking 100 g of glucose solution during measurement of OGTT (Tables 1 to 3).
| Patient Characteristics | Oral Glucose Tolerance Test | Independent sample t-test | P-value | ||||
|---|---|---|---|---|---|---|---|
| Normal (n=191) |
Gestational diabetes (n=9) |
||||||
| Mean± SD | Mean ± SD | ||||||
| Age (Years) | 24.53 ± 4.40 | 32.78 ± 8.18 | -3.004 | 0.016* | |||
| BMI (Kg/m2) | 24.68 ± 4.36 | 33.37 ± 5.77 | -5.765 | 0.000** | |||
| No. | % | No. | % | Chi square | P-value | ||
| Parity | Nulliparity | 85 | 44.5% | 0 | 0.0% | FE# | 0.000** |
| 1-2 | 72 | 37.7% | 1 | 11.1% | |||
| 3 or more | 34 | 17.8% | 8 | 88.9% | |||
(*) Statistically significant at P<0.05
(**) Highly statistically significant at P<0.01
(#) Fisher exact test was used as 20% of the cells or more have an expected count less than 5.
Table 1: Comparison between Group I (Gestational Diabetes) and Group II (Normal pregnancy) as regard age, BMI, parity and abortion.
| BMI (Kg/m2) | Oral Glucose Tolerance Test | Chi square | P-value | |||
|---|---|---|---|---|---|---|
| Normal (n=191) |
Gestational Diabetes (n=9) |
|||||
| No. | % | No. | % | |||
| Normal | 115 | 60.2% | 0 | 0.0% | FE | 0.000** |
| Overweight | 56 | 29.3% | 3 | 33.3% | ||
| Obese | 20 | 10.5% | 6 | 66.7% | ||
(#) Fisher Exact test was used as 20.0% of the cells or more have expected count less than 5
(**) Highly statistically significant at P<0.01.
Table 2: Comparison between Group I (Gestational Diabetes) and Group II (Normal pregnancy) as regard BMI categories.
| OGTT (mg/100 ml) | Age (Years) | BMI (Kg/m2) | ||
|---|---|---|---|---|
| OGTT (mg/100 ml) | Pearson Correlation | 1 | 0.412 | 0.399 |
| Sig. (2-tailed) | 0.000** | 0.000** | ||
| Age (Years) | Pearson Correlation | 0.412 | 1 | 0.315 |
| Sig. (2-tailed) | 0.000** | 0.000** | ||
| Sig. (2-tailed) | 0.111 | 0.203 | 0.061 | |
| BMI (Kg/m2) | Pearson Correlation | 0.399 | 0.315 | 1 |
| Sig. (2-tailed) | 0.000** | .000** | ||
(**) Highly statistically significant at P<0.01.
Table 3: Shows that there is a highly statistically significant positive correlation between OGTT and patient age, BMI (P<0.01).
Table 3 shows that there is a highly statistically significant positive correlation between OGTT and patient age, BMI (P<0.01).
Figure 1 shows that there is a highly statistically significant positive correlation between OGTT and patient BMI (P<0.01) (Tables 4 and 5, Figure 2).
| Uric Acid (mg/dl) | Oral Glucose Tolerance Test | Independent sample t-test | P-value | |
|---|---|---|---|---|
| Normal (n=192) |
Gestational diabetes (n=9) |
|||
| Mean ±SD | Mean ±SD | |||
| 3.07 ±.47 | 3.56 ±.83 | -1.760 | 0.115 | |
Table 4: Comparison between Group I (Gestational Diabetes) and Group II (Normal pregnancy) as regard uric acid.
| Uric acid (mg/dl) | Oral Glucose Tolerance Test | Chi square | P-value | |||
|---|---|---|---|---|---|---|
| Normal (n=191) |
Gestational diabetes (n=9) |
|||||
| No. | % | No. | % | |||
| Normal | 187 | 97.9% | 7 | 77.8% | FE | 0.025* |
| Abnormal | 4 | 2.1% | 2 | 22.2% | ||
(#) Fisher Exact test was used as 20.0% of the cells or more have expected count less than 5
(*) Statistically significant at P<0.05.
Table 5: Comparison between Group I (Gestational Diabetes) and Group II (Normal pregnancy) as regard uric acid.
Table 6 shows that there is a highly statistically significant positive correlation between OGTT and patient uric acid (P<0.01) (Figure 3).
| OGTT (mg/100 ml) | Uric acid (mg/dl) | ||
|---|---|---|---|
| OGTT (mg/100 ml) | Pearson Correlation | 1 | 0.197 |
| Sig. (2-tailed) | 0.005** | ||
| Uric acid (mg/dl) | Pearson Correlation | 0.197 | 1 |
| Sig. (2-tailed) | 0.005** | ||
(**) Highly statistically significant at P<0.01.
Table 6: Shows that there is a highly statistically significant positive correlation between OGTT and patient uric acid (P<0.01).
Table 3 shows that there is a highly statistically significant positive correlation between OGTT and patient uric acid level (P<0.01).
Figure 4 shows that Uric acid could significantly differentiate between cases of gestational diabetes and controls with an area under the curve=0.719 (Table 7).
| Positive if Greater Than or Equal Toa | Sensitivity | 1 – Specificity |
|---|---|---|
| 1.0000 | 1.000 | 1.000 |
| 2.0500 | 1.000 | 0.984 |
| 2.1500 | 1.000 | 0.979 |
| 2.2500 | 0.889 | 0.963 |
| 2.3500 | 0.778 | 0.948 |
| 2.4500 | 0.778 | 0.937 |
| 2.5500 | 0.778 | 0.859 |
| 2.6500 | 0.778 | 0.812 |
| 2.7500 | 0.778 | 0.791 |
| 2.8500 | 0.778 | 0.749 |
| 2.9500 | 0.778 | 0.670 |
| 3.0500 | 0.778 | 0.377 |
| 3.1500 | 0.778 | 0.335 |
| 3.2500 | 0.667 | 0.283 |
| 3.3500 | 0.667 | 0.215 |
| 3.4500 | 0.667 | 0.194 |
| 3.5500 | 0.667 | 0.141 |
| 3.6500 | 0.556 | 0.105 |
| 3.7500 | 0.556 | 0.089 |
| 3.8500 | 0.556 | 0.079 |
| 3.9500 | 0.333 | 0.052 |
| 4.0500 | 0.333 | 0.031 |
| 4.1500 | 0.333 | 0.021 |
| 4.2500 | 0.222 | 0.021 |
| 4.3500 | 0.111 | 0.005 |
| 4.7000 | 0.000 | 0.005 |
| 6.0000 | 0.000 | 0.000 |
| The test result variable(s): Uric_acid has at least one tie between the positive actual state group and the negative actual state group | ||
| a. The smallest cutoff value is the minimum observed test value minus 1 and the largest cut-off values are the maximum observed test value plus 1. All the other cut-off values are the averages of two consecutive ordered observed test values | ||
Table 7: Best cut off point to differentiate for Uric acid between cases of gestational diabetes and controls shows that the uric acid level of 3.1500 is the best cut off point that could differentiate between cases with gestational diabetes and normal controls with a sensitivity=77.8% and specificity=66.5%.
Table 6 shows that the Uric acid level of 3.1500 is the best cut off point that could differentiate between cases with gestational diabetes and normal controls with a sensitivity=77.8% and Specificity=66.5% (Table 8).
| Uric Acid | Preeclampsia | Chi square | P-value | |||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| No. | % | No. | % | |||
| Normal | 179 | 98.4% | 15 | 83.3% | 12.696 | 0.000** |
| Abnormal | 3 | 1.6% | 3 | 16.7% | ||
Relationship between preeclampsia and serum uric acid in the studied patients.
Table 8: shows that there is a highly statistically significant relationship between preeclampsia and serum uric acid (P<0.01).
Figure 5 shows that there is a highly statistically significant relationship between preeclampsia and serum uric acid (P<0.01)
Figure 5:Relationship between preeclampsia and serum uric acid in the studied patients.
It has been proven that, higher uric acid levels correlates with insulin resistance in women with hypertensive disorders of pregnancy and also higher levels of uric acid levels were noted at 24 to 28 weeks of gestation in women with GDM when compared to women without GDM [18]. Normally during pregnancy, the serum uric acid levels decreases significantly from 8th week of gestation up to 24 weeks due to increased glomerular filtration rate and decreased re absorption of uric acid from the renal tubules. In the first trimester, it likely approximates preconception uric acid level and elevated levels may identify women who are predisposed to metabolic syndrome with an increased risk of developing GDM. Using this concept, we aimed at a prospective analysis of association of first trimester elevated uric acid levels with the development of GDM. This would be useful in predicting GDM at an earlier gestational age, thereby aiding in appropriate management of the same to prevent maternal and fetal morbidity and mortality. In this current study, elevated first-trimester uric acid concentration was correlated with an increased risk of developing GDM. The risk of developing GDM was higher if first-trimester uric acid was <3.1 mg/dL. Mean blood sugar was found to be significantly highest among pregnant women with elevated serum uric acid and lowest among women with normal uric acid.
This result was similar in [19] who studied 1570 pregnant women uric acid was measured at mean gestational age of 8.9 ± 2.5 weeks and found that first trimester uric acid concentration <3.6 mg/dL (the highest quartile) were associated with a trend towards increased risk of developing gestational diabetes (adjusted OR=1.21, 95% CI: 0.75, 1.96) compared to women with concentrations below this concentration (lower three quartiles) [19].
These results were similar to that obtained by some studies. They had found that serum uric acid was significantly correlated with insulin resistance [20-22].
Our findings were consistent with Yoo et al. [23], who found, in a large cross-sectional study of 53.477 non-pregnant adults, that serum uric acid was positively correlated with fasting serum glucose and insulin resistance, as well as features of the metabolic syndrome, including waist circumference, low HDL cholesterol, hyper triglyceridemia, hypertension and fasting glucose <110 mg/dL [23]. However our study did not assess the other criteria of the metabolic syndrome.
Also, Choi et al. [24] found that uric acid in the first trimester likely approximates pre-conception uric acid, and elevated uric acid may identify women who are predisposed to metabolic syndrome with an increased risk of developing GDM, independent of obesity. Alternatively, uric acid decreases early in pregnancy, so perhaps women with elevated uric acid decreases early in pregnancy, so perhaps women with elevated uric acid have a poor adaptation to pregnancy (i.e., abnormal placentation), putting them at risk for adverse pregnancy outcomes such as GDN [24] .
In contrast to Gungor et al. [25], who compared the relationship between serum uric acid, creatinine and albumin levels in pregnant women with normal glucose tolerance and gestational diabetes mellitus. A total of 112 patients were evaluated, 56 of whom had gestational diabetes. All of the patients had single estimations of serum uric acid, creatinine, albumin and liver enzymes carried out on booking between 24-28 weeks gestation. The women were followed up throughout pregnancy. They found that single estimations of serum uric acid levels were higher in the diabetic patients, but this elevation was not statistically significantly and also albumin concentrations were not significantly different between a normal pregnant group and a GDM group [25], which is contrary to the of findings of our study, this may be due to the differences in the number of cases and gestational age of serum uric acid estimations.
But in the same prospective study of Turkish women Gungor et al. [25] found that creatinine levels were significantly higher in the diabetic group than in the control group, which is similar to our study. In our study, we found that creatinine levels were significantly higher in the diabetic group than in the non-diabetic group [0.6+/-0.098 vs. 0.58+/- 0.148 mg/dL with significant difference (p>0.050) [25].
Also, in this current study, women who developed GDM were significantly older when they compared to women who did not develop GDM [Normal with Mean+SD 24.53+4.40 years, Abnormal with Mean+SD 32.78+8.18 years, p-value 0.016], this agrees with Lao et al. [26], in Queen Mary Hospital, The University of Hong Kong, who have reviewed the prevalence of GDM, diagnosed by the world Health Organization criteria in over 15000 singleton pregnancies managed from 1998 to 2001.
The pregnancies were categorized according to maternal age, into 6 categories, >20 years, 20-24 years, 25-29 years, 30-34 years, 35-39 years and
There was no significant difference for the >20 years, 10.85 (7.72- 15.25) and
In this study, the mean BMI was significantly higher in women who developed GDM when compared to women who did not develop GDM (Normal 115 cases of total sample and no one developed GD with Mean+SD, 95.57+12.32, Overweight 59 cases, 56 cases (29.3%) were normal and 3 cases (33.3%) had GD with Mean+SD 106.29+26.62, Obese 26 cases, 20 cases (10.5%) were normal and 6 cases (66.7%) had GD with Mean+SD 124.27+39.78, p-value 0.000), this agrees with Loa et al. [26] studied 1121 patients with GDM who were referred to the outpatient clinic of Szczecin University in Poland between January 2001 and December 2005. The control group consisted of 1011 healthy pregnant women. All had singleton pregnancies. Significant relationships between pregravid BMI and GDM treated with insulin. This study states the same results as our study.
In another study, Jenny et al. [27], who studied 1733 patients with singleton pregnancies enrolled in project viva. They examined the associations of first trimester diet, with results of glucose tolerance testing at 26-28 week’s gestation. 91 patients developed GDM and 206 patients had impaired glucose tolerance (IGT). They concluded that pre-pregnancy body mass index (BMI) is a strong predictor for development of GDM [27]. This study states the same results as our study.
In this study, it was found that, the women who developed GDM had significantly more number of children (Nullipara were 85 cases (44.5%) of total sample size and no one had GD with Mean+SD 97.61+13.20, 1-2 children were 73 cases of total sample size, 72 cases (37.7%) were normal and 1 case (11.1%) had GD with Mean+SD 98.23+16.87, 3 or more were 42 cases of total sample size, 38 cases (17.8%) were normal and 8 cases (88.9%) had GD with Mean+SD 119.62+40.08) with p-value 0.000, also we found a relationship between elevated serum uric acid level and the development of preeclampsia (18 cases developed preeclampsia, 15 cases with normal serum uric acid (83.3%) and 3 cases had elevated serum uric acid (16.7%)) and (182 did not develop preeclampsia, 179 cases with normal serum uric acid (89.4%) and 3 cases had elevated serum uric acid (1.6%)) with p-value 0.000 this agrees with Bener et al. [28], in a prospective cohort study carried out at the antenatal clinics of the Women’s Hospital Qatar. 2056 pregnant women were surveyed during the period from January 2010 to April 2011. They found that the prevalence of GDM in Qatar was 16.3% and GDM was significantly higher in the age group of 35-45 years (45%, p=0.001). Increased parity (55.3%, p=0.004), and obesity (59.2%, p, 0.001) were Maternal complications like pregnancy-induced hypertension (19.1% vs. 10.3, p, 0.001), pre-eclampsia (7.3% vs. 3.8%, p=0.012), antepartum hemorrhage (19.2% vs. 14.6%, P=0.05) and cesarean (27.9% vs. 12.4%, p0.001) were significantly higher in GDM women. Neonates were at increased risk of preterm birth (12.6% vs. 8.3%, p=0.03), macrosomia (10.3% vs. 5.9%, p=0.01) and birth trauma (8% vs. 3% p=0.001). The study findings revealed that GDM was higher in women in Qatar and that they were at increased risk of developing maternal and neonatal complications. Obesity emerged as an essential risk factor for subsequent GDM. The advanced maternal age, low monthly income, family history of advanced maternal age, low monthly income, family history of diabetes and obesity were the main significant risk factors for GDM [28], which are similar to the results of our study. However our study did not assess the prevalence of GDM.
Another study by Teh et al. [29] at Monash Medical Centre, Victoria, Australia, a tertiary obstetric referral hospital, analyzed the pregnancy outcomes for the year 2007. Data of singleton pregnancies in women (without pre-existing diabetes mellitus) were routinely entered into an electronic database, the Birthing Outcomes System (BOS), at the first booking antenatal visit, throughout antenatal care and at the time of birth. All women underwent GDM screen with a non-fasting 75 g glucose challenge test (GCT) at 26-28 weeks’ gestation. Women with a positive result (1 h venous plasma glucose level <8.0 mmol/L) proceeded to a 2 h 75 g oral glucose tolerance test (OGTT). GDM was diagnosed in the presence of either a fasting venous plasma glucose level of <5.5 mmol/L or a 2 h level of <8.0 mmol/L. They found that the strongest independent risk factors for GDM were a past history of GDM (OR=10.7, 95% CI: 5.4-21.1), maternal age <40 years (OR 7.0, 95% CI: 2.9-17.2) and BMI<35 kg/m2 (OR 6.1, 95% CI: 3.0-12.1) They concluded that increasing age and BMI and previous GDM were the most significant risk factors for GDM [29], which are consistent with results of our study.
Elevated first-trimester uric acid concentration was correlated with an increased risk of developing GDM.
So we recommended to:
• Increase the number of study sample.
• The study should focus on pregnant women at risk.
• Retrospective study is more accurate and saves money and time.
• Make a study to determine the effect of elevated serum uric acid on blood pressure and the risk of development of gestational hypertension and preeclampsia.