Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Review Article - (2020)Volume 16, Issue 1
Most of the clinically available monoclonal antibody (mAbs) drugs are Immunoglobulin G's (IgG's). The variability of the IgG subclasses is in the amino acid content of the hinge region which forms the basis of their stability and suitability for therapeutics development. Monoclonal antibody drug development is a tedious and long-term process requiring putting many factors into consideration. The variability in the stability, flexibility, mediation of antibody dependent cell cytotoxicity (ADCC), mediation of cellular dependent cytotoxicity (CDC), and C1q protein binding are major factors that determine the suitability of IgG subclasses for the development of therapeutics. It was reviewed that most of the marketed mAbs therapeutics are IgG1 subclass, this is due to its stability and less aggregate formation, triggering of effector function via the action of Fc domain binding to FcyRI, FcyRII, and FcyRIII, resulting to mediation of ADCC, CDC, and C1q cascade of signaling. However, IgG2 is also utilized for the development of therapeutic when neutralization of soluble antigen with reduce effector function is required, with some drugs in late stage development and also approved for commercial use. Also, IgG4 is utilized for the development of therapeutics drugs when the recruitment of the host effector function is not required. But IgG3 utilization for the development of therapeutics requires engineering of the amino acids content of the hinge region, without any commercially available drug that is IgG3. This review examines the suitable IgG subclasses with the capability of ADCC, CDC, and C1q mediation, and also provides future recommendation on the suitability of less stable IgG subclasses in the therapeutic development.
Immunoglobulins; Immunoglobulin G; Immunoglobulin G subclasses; Monoclonal Antibodies; Hybridoma Technology
The variation in stability of different types of immunoglobulin G sub classes affect their suitability in the development of therapeutic monoclonal antibodies, the subject of discussion in this review is the best immunoglobulin G for the development of therapeutics monoclonal antibodies. Therefore, it is important to have a little discussion about Immunoglobulins (Table 1). The complex protein molecule called immunoglobulin are the widely studied protein before the invention of automated protein sequencing and recombinant DNA technology, their application as disease therapeutics date back to their detailed knowledge from structure, immunology, biology, synthesis of large quality biopharmaceuticals. Careful structural and biological investigation using x-crystallography and amino acid sequencing into the protein crystal in the urine of B-lymphocytes cancer infected patient gives scientist an in-depth insight in understanding of abundance and stability of immunoglobulins that confers immunity to various infectious pathogens. The stability of this biomolecule is attributed to it physical property such as inter and intraspecific disulphide bond, and diversity of antigen recognition by antibody makes this molecule target for therapeutic development [1]. The history of IgG date back to 1790s with the discovery of protective substance in the serum following vaccination against small pox disease. This protective substance was now known as an immunoglobulin which are glycoproteins called antibodies produced by plasma cells, they mediate immunity by specific binding to an antigen. The host immune system comprises of distinct cells such as Langerhans, dendritic cells, basophil, mast cells which work with lymphocytes to mediate response. The two classes of lymphocytes are B and T, the T enhancement of phagocytosis and viral infected cells lysis. The B- lymphocytes secretes immunoglobulins which are antitoxin neutralizing molecules [1]. There are various classes of immunoglobulins each differs in their size, shape, biological role, abundance/distribution, target specificity and half-life. The various classes are (Table 1) immunoglobulin A (IgA), immunoglobulin D (IgD), immunoglobulins E (IgE), immunoglobulin G (IgG) and immunoglobulin M (IgM). The IgA is the antibody produced in mucus [2] cells such as respiratory track, gastrointestinal track and urogenital track that prevent colonization of pathogens. The IgD is the antibody produce on the surface of B-cells that serve as receptor for antigen for cells that are not exposed to antigen [3]. This antibody is responsible for the production of antimicrobial factor in mast cells and basophils [4]. IgE is the antibody that respond to allergen and produce histamine from mast and basophils. The IgM therefore is antibody produced and secreted on the surface of B-cells that complement B-cell immunity before sufficient IgG is produced and secreted into the plasma [5].
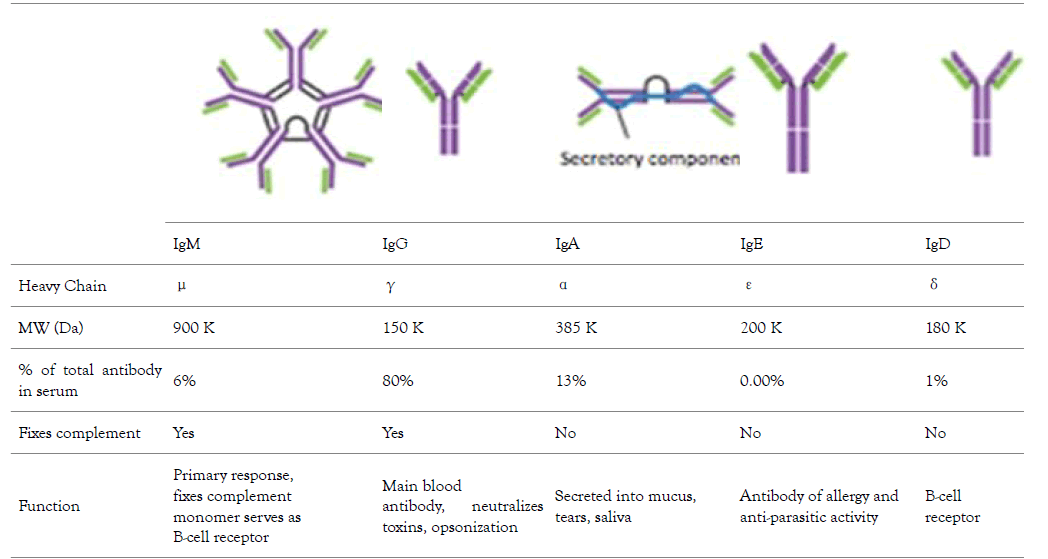
Table 1: The different types of human Immunoglobulin, their abundance in serum, molecular weight, type of heavy chain present, and their biochemical function [6].
There are different mechanisms of immunity mediate by IgG molecule such as ADDC, CDC and C1q pathway. Antibodydependent cellular cytotoxicity (ADCC) (Figure 1) is a set of mechanisms that target antibody (IgG) covered cells to be the prey of cell-to-cell cytolysis carried by immune cells expressing FcRIIIA (CD16A). The effectors include natural killer (NK) cells, and also other CD16+ subsets such as monocyte/ macrophages, NKT cells or γδ T cells [7]. The specificity of the killing is determined by the specificity of the involved IgG subclass antibody and not the antibody-dependent cell-mediated cytotoxicity effector cells. Certain type of IgG subclasses mediates the killing of the coated cells [8]. Cell mediated cytotoxicity (Figure 1) is a process by which the immune system i.e. the antibody – antigen complex activates a cascade of proteolytic enzymes that ultimately results in the formation of a terminal lytic complex that is inserted into a cell membrane, resulting in lysis and cell death [9]. In C1q protein binding of antibody to an antigen results to formation of antigen antibody complex followed by binding of C1q protein which will results to activation of the complex, and then triggers complement pathway which result generation of C3 convertase that cleave C3 protein, the cleaved protein binds to C3 protein to form C5 convertase resulting to phagocytosis of the infected cells and membrane attack complex activation (MAC) which cause lysis and death of cells [10]. This review highlight the function of IgG subclasses in the mediation of immunity, provides an insight into their stability, and emphasize on their suitability for therapeutic development.
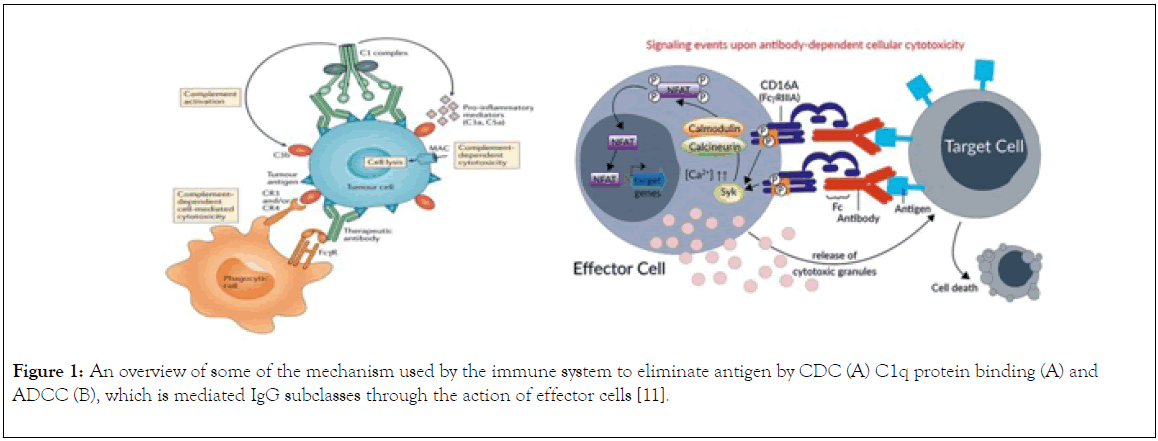
Figure 1. An overview of some of the mechanism used by the immune system to eliminate antigen by CDC (A) C1q protein binding (A) and ADCC (B), which is mediated IgG subclasses through the action of effector cells [11-14].
The IgG (Figure 2) is a polymeric protein with size of 150KDa (2 heavy chains accounting for 100KDa and two light chains accounting for 50KDa) that contains two gamma heavy chains and light chains held together by four disulphide bridges, the two heavy chain are subdivide into domains, these domains are variable domain (VH), and three constant domains (CH1, CH2, CH3), the light chain of IgG is divided into two domains, the variable domains (VL) and constant domain (CL). Both the H and L chains are joined by disulphide bond in a region called the hinge region (Figure 2).
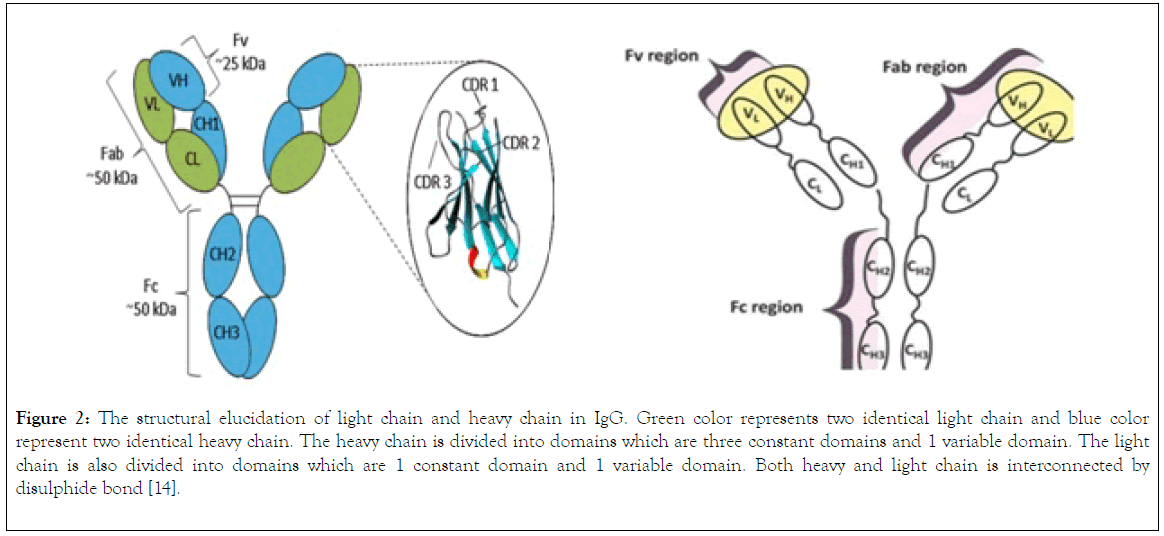
Figure 2. The structural elucidation of light chain and heavy chain in IgG. Green color represents two identical light chain and blue color represent two identical heavy chain. The heavy chain is divided into domains which are three constant domains and 1 variable domain. The light chain is also divided into domains which are 1 constant domain and 1 variable domain. Both heavy and light chain is interconnected by disulphide bond [14].
The complementary determining region (CDR) which is located within the variable domain of both heavy and light chain (Figure 2) determines allotypes, idiotypes, antigen binding selectivity, antibody affinity to an antigen, cellular function and cell mediated complement induced cytolysis and antibody dependent cellular function. The antigen binding site is contributed by Fv region and Fab region (Figure 2), these sites are used by the antibody to bind the antigen, also the binding of antibody to a receptor such as Fc gamma receptor on the surface of macrophages is mediated by Fc (Constant domain 3 and 4 of the two-heavy chain) region of the IgG thereby enable the mediation of phagocytosis of antigen [12].
The components of immunoglobulin G are divided into three different regions, that is variable fragment (Fv), antibody binding domain (Fab) and crystallisable domain (Fc) (Figure 2). Glycosylation (Figure 3) take place in the CH2 of the two-heavy chain, which is required for the antibody stability, complement dependent cytotoxicity, antibody dependent cytotoxicity by modulation of binding to gamma receptor [13,14], a specific glycoforms of sugar are necessary to achieve efficacy, glycoforms can be achieved and targeted by glycol engineering which involve the alteration of CH2 domain. Heavy chains are synthesized from 4 distinct gene coding for variable domain (VH), constant domain (CH), joining region and diversity region. Also, the light chain is encoded by three different genes such as variable domain gene (VL), constant domain gene (CL). This is to say immunoglobulin is synthesized by multiple genes coding for different domains (Figure 4) [15].
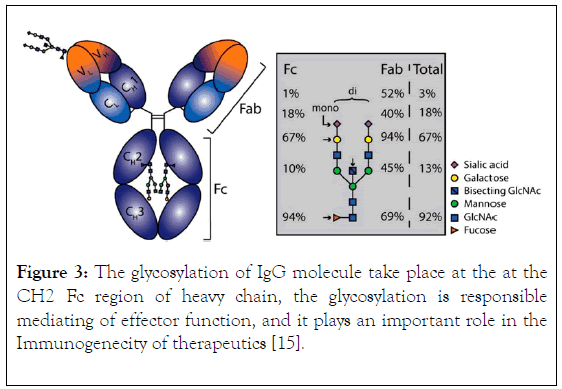
Figure 3. The glycosylation of IgG molecule take place at the at the CH2 Fc region of heavy chain, the glycosylation is responsible mediating of effector function, and it plays an important role in the Immunogenecity of therapeutics [15].
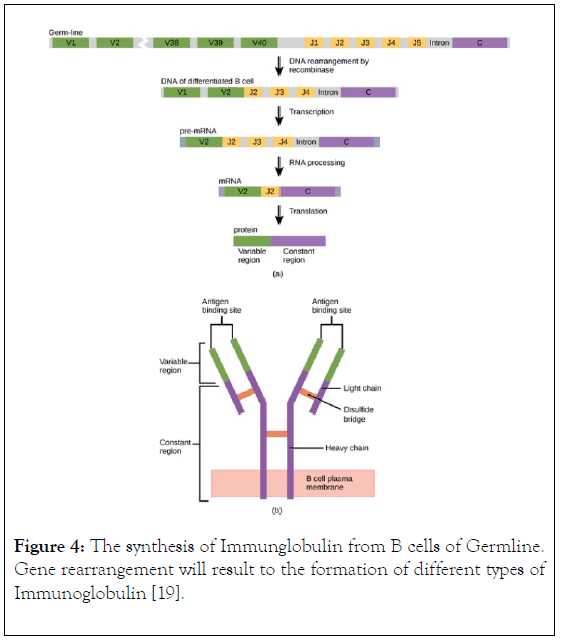
Figure 4. The synthesis of Immunglobulin from B cells of Germline. Gene rearrangement will result to the formation of different types of Immunoglobulin [19].
An individual is capable of generating IgG against an unlimited number of antigens but human do not have sufficient genes that encode IgG for all antigen, however, antibody diversity is the cellular mechanism used by B-cells to produced unlimited number of antibodies, this can be achieved by structural gene combination or somatic mutation (Figure 4). Studies have shown that heterogeneity of immunoglobulin classes is mainly attributed by amino acid variability at the amino terminal part of light and heavy chains, other part of immunoglobulin within a given class have a common structure and composition [16]. Upon infection with a pathogen the B-cell produce IgG which bind to the antigen using it antigen binding site located in both the variable domain, after binding, the antibody binds to a gamma receptor on the surface of macrophages to mediate internalization of the antigen into macrophage followed by destroying of the antigen with the help of molecules produced by T-cells, therefore, mediating adaptive and innate immunity.
This type of antibody has numerous advantages such as predominating the control of antigen by either phagocytosis, pathogen clearance, inflammation resolution in the blood and other abundance of IgG also occurs in cerebrospinal fluid and peritoneal fluid therefore playing a key role in mediation of Humoral immunity, conferring of protection to foetus since It can bypass the placenta, it is the antibody that gives protection to newly born babies before their immune system is fully developed, IgG can migrate all tissues spaces including peritoneal fluids, cerebrospinal fluid, the half-life of IgG is 23 days, having more life span than the other classes of immunoglobulins in the serum before it clearance [17].
Therefore, the higher the life span the longer term of immunity that will be provided by an antibody [18]. the IgG is the highly abundant antibody accounting for 75% of the total antibody present in the serum, the rest of the percentage are accounted by IgA, IgE, IgD and IgM, the aforementioned features of IgG makes it to be utilized for therapeutic development compared to other Immunoglobulins.
Immunoglobulin G subclasses differs in their ability to mediate effector response, which give them variation in responding to either tumours or microbes [20]
IgG is divided into four different subclasses (Figure 5), and the source of variation in each subclass ranges from serum concentration, molecular form, functional valency, serum halflife, activation of classical pathway, crossing of placenta, binding to Fc receptor, number of disulphide bond in the hinge region [18]. The variability of Fc and hinge region of the subclasses of IgG correlate with their ability to bind to inhibitory and activating receptor of FcYc, complement component C1 and neonatal receptor for immunoglobulin G. Also, the variation in the hinge region gives each and every subclasses of IgG different unique physical and structural character such as distance spanned by Fab and Fc, flexibility, stability [13]. This variation in the subclasses of IgG forms the basis of their selection for antibody based therapeutic purposes. Declining in the concentration of Immunoglobulin G is associated with certain chronic infection, making them biological markers for clinical diagnosis [21].
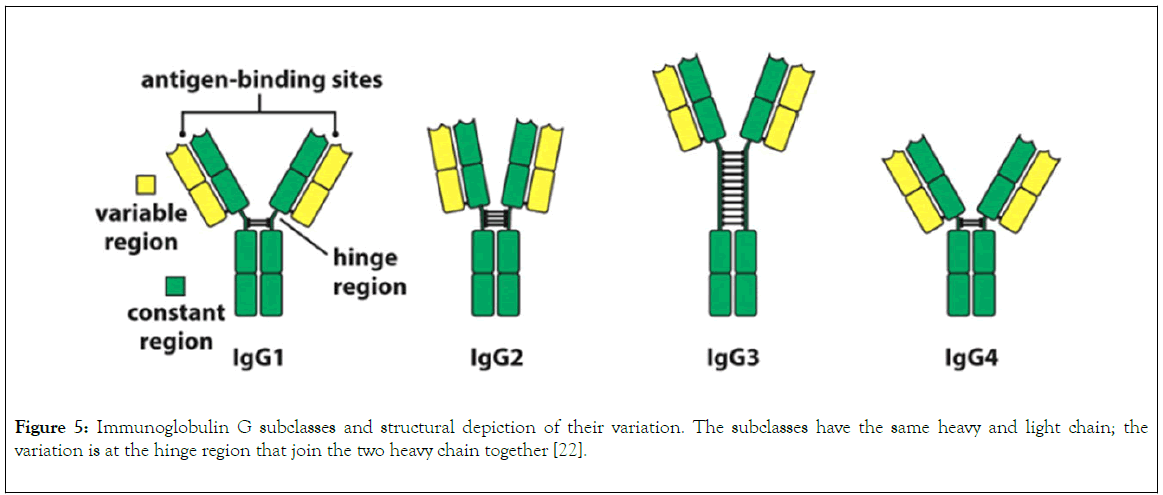
Figure 5. Immunoglobulin G subclasses and structural depiction of their variation. The subclasses have the same heavy and light chain; the variation is at the hinge region that join the two heavy chain together [22].
Immunoglobulin G1
IgG41 are induce upon response to soluble protein antigen and associated membrane protein, this response to protein antigen is accompanied by other IgG subclasses such as IgG3 and IgG4. IgG1 is the predominant antibody in the serum, deficiency of this subclass is associated with fall in total IgG in the serum, a clinical condition called hypogammaglobulinemia. Clinically, deficiency of IgG1 is associated with recurrent infection [23,24].
Immunoglobulin G2
This subclass respond to bacterial capsular polysaccharide in which deficiency in this antibody is associated with absence of anti-polysaccharide, the bacterial response can be compensated by alternative subclasses such as IgG3 and IgG1. However, an individual with such deficiency is likely to be prone to bacterial infection. In addition, IgG2 deficiency is associated with IgG1 deficiency [25]. Investigation has shown that IgG2 is highly reactive against glycan, this was carried out by investigating anti glycan reactivity in intravenous immunoglobulin. IgG2 antibodies have also been reported to prevail against Haemophilusinfluenzae b polysaccharide during natural infections [26].
Immunoglobulin G3
Effector function mediated by this subclass of IgG is particularly in the induction of anti-inflammatory action. Surprisingly, the level of IgG3 does not always increased which may be due to polymorphisms that affect the frequency of class switching to IgG3 in G3m (g) allotypes, this occurs in homozygous individual [27]. Upon infection with virus, both IgG3 and IgG1 are triggered, but IgG3 appears during the early stage of infection. However, with the progression of the infection, IgG1 predominate IgG3 [28]. Also, response that is associated with pregnancy and blood transfusion are associated with IgG1 [29,30]. Therefore, fall or absence of IgG3 in the serum is associated with deficiency of other associated IgG subclasses [31].
Immunoglobulin G4
IgG1 and IgG4 are also induced by allergens, in addition to IgE. The formation of IgG4 antibodies are often following repeated and long-term exposure to antigen in a non-infectious setting and may become the dominant subclass [19]. The dominant response to therapeutic proteins like factor VIII and IX is mediated by IgG4. In addition, helminth parasite infections may result in the formation of IgG4 antibodies, and high IgG4 titres can be associated with an asymptomatic infection [31]. A group of disorders nowadays referred to as IgG4-related diseases (IgG4RD) are characterized by elevated serum IgG4 concentration and tissue infiltration by IgG4-positive plasma cells and may affect a number of organs [32,33].
The Best IgG Subclass for Therapeutics Production
Effector functions for the different IgG classes are directly correlated with their affinity for the Fc receptor, with IgG1>IgG3>IgG4>IgG2 [34]. Ryman and Meibon report that as the IgG is sub divided into four subclasses which are IgG1, IgG2, IgG3, and IgG4, Typically, IgG1 and IgG3 are potent triggers of effector mechanisms, whereas IgG2 and IgG4 will induce more subtle responses, and only in certain cases [35]. However, each of these antibodies remain capable of neutralizing target antigens. Currently marketed mAbs are predominantly IgG1, with a lesser degree of IgG2 and IgG4. The preference for one IgG class over the other is partially determined whether effector functions, such as antibody dependent cellular cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC), are desired for the mAb activity as well as other structural factors. But also, by prior experience and availability of a particular IgG subclass in a company ’ s development portfolio. As reviewed by Rodney, the most abundant IgG subclasses in the serum are IgG 1, 2 and 4 because of their metabolism in the serum take place at a slower rate than IgG 3, as a result of their persistence in the plasma [36]. Hence, IgG 1, 2 and 4 are used clinically for chronic condition but not IgG 3 because the hinge region of this antibody subclass is highly unstable as a result of higher number of hinge disulphide bonds than IgG 1,2 and 4 which make IgG 3 more prone to aggregation in solution which might affect it therapeutic potential. Also shorter serum half-life (arginine is present in IgG1, 2, 4 but in IgG 3 histidine replaces arginine at 435 amino acid position resulting to inhibition of IgG3 by IgG1 from binding to FcRn resulting to breakdown of IgG 3 rather than recycling), higher molecular weight, less passage to placenta makes IgG3 not suitable for drug development. Among those three frequently used IgGs (1, 2 and 4) for therapeutics, the most frequently used is IgG 1 which is due to the fact that the hinge region is more stable than IgG 2, the disulphide bond of IgG 1 is the same with that of IgG 4 i.e. It is also stable, what makes IgG4 less utilized for therapeutics than IgG 1 is its least serum concentration and percentage, IgG 1 do not aggregate which is also a good feature for its selection as therapeutics [13]. Furthermore, the most frequently chosen IgG isotype for the development of therapeutics is IgG1 [37]. This is accounted by the Fc domain of IgG1 molecule to trigger effector function by its ability to bind to Fc receptor (FcγRI, FcγRII or FcγRIII), the C1q component of the complement pathway. FcyRs mediates Effector functions such as ADCC (antibody dependent cell mediated cytotoxicity), the CDC (complement dependent cytotoxicity) action occurs via binding to the C1q protein, interaction of IgG with neonatal Fc receptor (FcRn) is the reason why the half-life of IgG is higher in the serum [38-40]. IgG 1 contains a total of 16 disulfide bonds (4 inter-chain and 12 intra-chain). ER is the compartment where correct pairing of the 16 disulphide occurs which is aided by chaperones and protein disulphide isomerase. But occasionally IgG1 molecules might have a population of molecules where the intra-chain disulfide bond in the VH domain between Cys22-Cys96 residues is not paired [41,42]. Recent important finding by Ouellette is that serum exposure of the unpaired cysteine residues of the mAb will result to reformation of their disulfide bond. Rapid pairing of the cysteine residues of IgG1 was observed in serum from analytical evaluation of the mAb recovery from the serum, this might be that thiol group present in the serum deliver good environment outside the ER, and this is in contrast to IgG2 and 4. Therefore, good disulfide pairing is good feature for antibody to bind to an antigen [43]. However, IgG2 is chosen for the development of therapeutics when the aim is to neutralize soluble antigen with reduced effector function, also, it is at its late stage development. Detection of fully functional covalent dimers in recombinant antibodies secreted myeloma cells and normal donor sera, is a unique charctersitics of IgG2 [44]. There is low affinity Interaction between IgG2 and antigen at the carbohydrate site of the antigen [45]. IgG2 activity in the serum is modulated by disulphide shuffling in the serum, which is an interesting characteristics of IgG2 molecule [46-48]. Interestingly, IgG2 exist in three isoforms i.e. IgG2A, IgG2A/B, and IgG2B, their formation take place in the serum by conversion from A to A/B then to B which takes many days and is associated with the reduction of the activity of IgG2 molecule this is brought about by the restriction of movement of Fab region of the molecule. IgG2 showed decrease in in activity compared to IgG1 [49]. IgG 4 is a tetrameric molecule containing two region cysteines, even though that this molecule is similar structurally to IgG 1, half of IgG4 antibody is composed of HL dimers [50,51]. As reviewed by Correia, In contrast to IgG1, IgG4 molecules have a low affinity for C1q; therefore, recombinant IgG4 molecules have emerged as an important therapeutic class of molecules when recruitment of the host effector function is not desirable [52]. Example of the currently marketed antibody therapeutic that are IgG4 are Natalizumab (Tysabri) and gemtuzumab ozogamicin (Mylotarg) for multiple sclerosis (MS) and acute myeloid leukemia (AML). IgG4 molecule undergoes endogenous Fab arm exchange with another IgG4 molecule, this is basically normal endogenous biological process. However, this biological process is not desirable in therapeutics antibodies and it can affect pharmacokinetics, pharmacodynamic and cause other adverse effect upon consumption of the drug. For example, Fab exchange was observed between TGN1412 and endogenous IgG4 molecule in an individual treated with the drug. However, mutation of CH3 region of the fab can solve this problem, in which mutation of gemtuzumab ozagamycin did not result to Fab arm exchange as shown in studies carried out with mice [53]. In contrast to IgG1,2 and 4, as a result of IgG3 subclass high allotypic polymorphism, susceptibility to proteolysis owing to the long hinge region, and short half-life in the body, that is why it receives low attention in therapeutic development, this class of the antibody can easily form aggregate which makes it prone to causing of immunogenicity, but engineering of the CH3 region of IgG3 molecule will allow it to be explore for the production of therapeutics monoclonal antibody. Findings reported by Saito showed that engineering of CH1, hinge region, CH2 and CH3 of IgG3 by replaying the corresponding domains with that of IgG1, it has an effect on the stability of IgG3 molecule, replacing of CH1 of IgG3 with that of IgG1 will result to formation of 1133 engineered IgG3, and subsequently replacement of hinge region, CH2, CH3 of IgG3 with that of IgG1 will result to formation of 3311, 3313, 3331 engineered IgG3 respectively, therefore, hinge region and CH3 domain engineering of IgG3 reduce the formation of aggregates, but CH1 and CH2 engineered antibody show formation of aggregated as the wild type IgG3 (Figure 6), this is to say that CH3 and hinge region are the two domains that affect the aggregation formation by IgG3. He also proved that IgG3 can bind to a FcyRIII and mediate ADCC, this is supported by Saito, he reported that IgG3 bind with high affinity than IgG1 (Figure 7), IgG3 can also bind to C1q protein and mediate complement pathway activation (Figure 7), this study was carried out using ELISA.
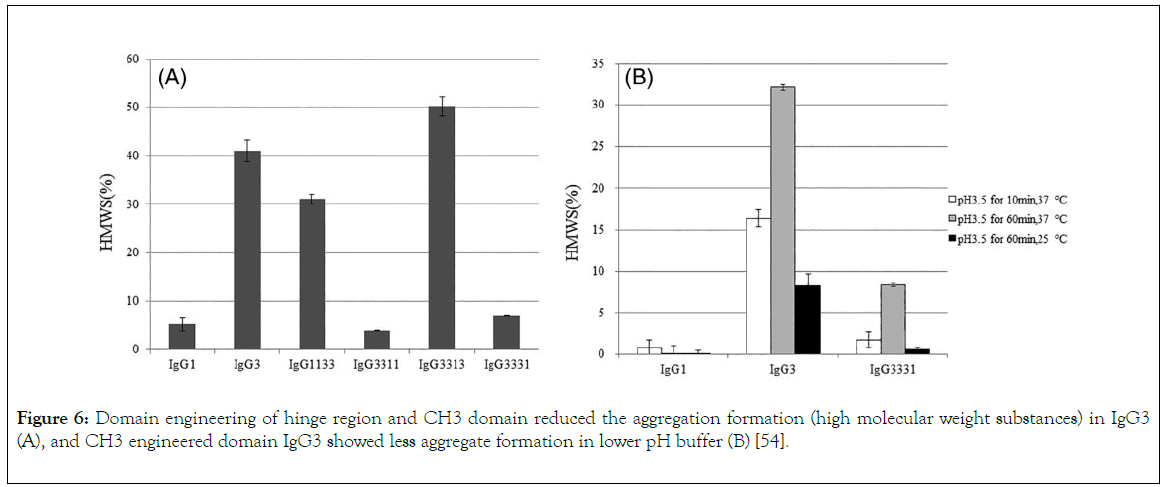
Figure 6. Domain engineering of hinge region and CH3 domain reduced the aggregation formation (high molecular weight substances) in IgG3 (A), and CH3 engineered domain IgG3 showed less aggregate formation in lower pH buffer (B) [54].
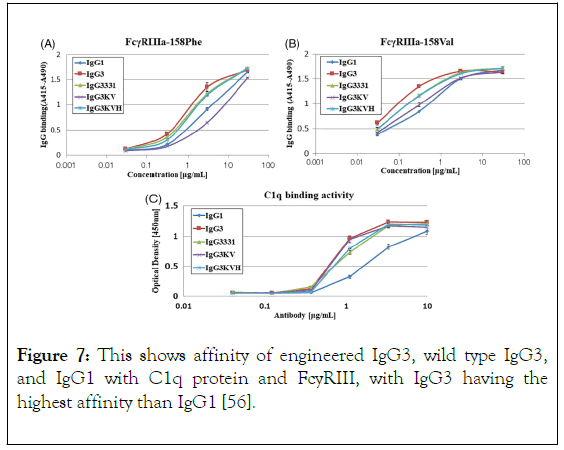
Figure 7. This shows affinity of engineered IgG3, wild type IgG3, and IgG1 with C1q protein and FcyRIII, with IgG3 having the highest affinity than IgG1 [56].
IgG3 subclass antibodies exhibit high antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) compared with IgG2 and IgG4. The unique character of IgG3 arises from a 62-amino acid long hinge region that confers a high flexibility on the Fab arm, which in turn influences the antigen-binding ability [19]. For example, human IgG3 monoclonal antibodies targeting HIV have a higher neutralizing ability than the IgG1 subclass. As shown by some studies, IgG3 correlates with a lower risk of HIV-1 infection and other infectious diseases. IgG3 antibodies do have the ability to efficiently opsonize red blood cells or microorganisms and induce a superior phagocytosis. These unique characteristics indicate that human IgG3 will provides a new platform for developing therapeutic antibodies against several diseases [55].
The aforementioned discussion is based on the differences of IgG subclasses on complement fixing and FcyR binding. The following discussion will be based on the differences of IgG subclasses flexibility. According to Tian the relation between flexibility of IgG subclasses and effector function remain unclear, for example, IgG4 adapts to a preferred Y/T-shape, which implies a possible steric hinderance on one C1q binding site when the antibody adapts to this conformation. However, Xu et al. reported that a single mutation (S331P) in the CH2 domain of IgG4 can restore approximately 50% of complementbinding ability compared with IgG1 [57,58]. There is thus a complex relationship between flexibility and activity. The primary structure and the preferred conformation or flexibility of antibodies might have collective effects on the effector functions, where the primary structure is expected to play the determinant role, but further studies are needed to elucidate the importance of the different parameters. As an example, it is well known that IgG2 and IgG4 are more prone to aggregation than IgG1 [59]. It has been demonstrated above that IgG2 has a dominant Y-shape and IgG4 has alternating conformational shifts between a Y-shape and a preferred Y/T-shape in solution, while IgG1 reveals a much greater flexibility, accommodating almost one third of T-shaped conformations. A number of aggregation-prone motifs of IgG1 have also been identified most of which are concentrated around the lower hinge region. In addition, these motifs are largely preserved in the primary sequences of all IgG subclasses. Thus, it was suggested that the T-shape might shield the aggregation-prone motifs of IgG1 and improve its physical stability [60]. A second possible link between the observed structural ensembles and antibody stability is based on the reported differences in fragmentation for IgG subclasses. IgG1 is more susceptible to non-enzymatic cleavage [61]. As several active cleavage sites have been identified in the upper hinge region of IgG1, it was anticipated that the flexibility and solvent exposure of this region also influence fragmentation [62]. However, the overall fragmentation susceptibility is likely to be determined by collective effects of both primary and tertiary structure. In addition, Table 2 summarises the properties of IgG subclasses that influences their selection for therapeutics as reported by [18]. The study of antibody stability and conformation can be carried using Ultracentrifuge which can give information about the heterogeneity of the protein under different storage condition [63]. Investigation of IgG1, 2 and 4 using small angle X-ray scattering reveals that the difference is reflected by pair distance distribution function generated from indirect fourier transformation scattering data (Figure 8a and b), and IgG1 is slightly higher in dimension than IgG2 and IgG4 (Figure 8C) which is due to largest values for radius of gyration (Rg) and volume correlation.
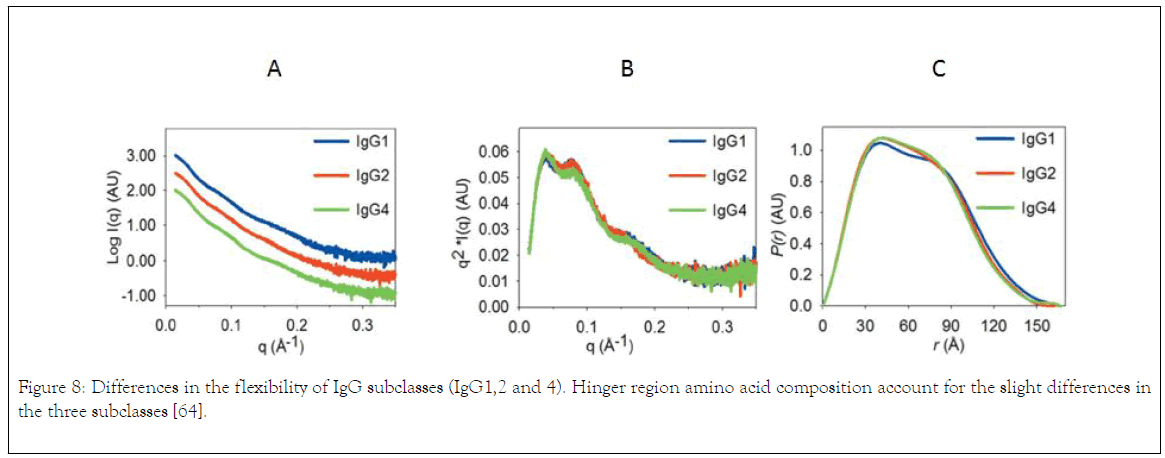
Figure 8. Differences in the flexibility of IgG subclasses (IgG1,2 and 4). Hinger region amino acid composition account for the slight differences in the three subclasses [64].
| Property | IgG1 | IgG2 | IgG3 | IgG4 |
|---|---|---|---|---|
| Serum concentration (mg/ml) | 44170 | 43984 | 0.5-1 | 0.2-1 |
| Serum half life | 21-24 | 21-24 | 44050 | 21-24 |
| Molecular weight (KDa) | 150 | 150 | 160 | 150 |
| Affinity to Fc gamma receptor | Strong affinity | Partial affinity | Strong affinity | Partial affinity |
| % in serum | 45-53 | 42309 | 43985 | 43922 |
| Complement activation | + | +/- | ++ | - |
| Placenta crossing | Yes | Yes | No | Yes |
| Drugs Commercially available | Adalimumab Alemtuzumab | Denosumab Evolocumab | Non | Duplumab Nivolumab |
Table 2: The Properties of IgG subclasses that determine their suitability for the development of therapeutics monoclonal antibody [18].
Development large scale commercial manufacturing of monoclonal antibodies is as a result of increase in the demand of therapeutics with the number of therapeutics approved for use continuous to increase over the years. Modifications of the development of monoclonal antibodies has led to improvement of the use of mAbs in various forms of therapeutic applications such as treatment of infectious diseases caused by bacterial, viral, fungal and parasitic organisms. Monoclonal antibodies have also been applied in the treatment of non-infectious diseases such as cancer, immune diseases, arthritis and other disorders resulting from organ transplantation [63]. The steps for the development of therapeutic monoclonal antibodies are as shown in the following discussion.
Mice immunization with a specific antigen
Specific antigen injection through antigen emulsification with adjuvant, and gel slice homogenization containing the antigen. The immunization is normally done after two weeks in which when sufficient amount of antibody is reached, the animal is then sacrificed, and the spleen can be collected for lymphoid organ. Spleen is then subjected to enzymatic or mechanical action which result in the release of B cells, the activates B cells of the plasma cells are separated from the spleen B cells by a technique called density gradient centrifugation. Example of antigen that can used are Virus and Bacteria.
Screening for monoclonal antibody production
During weekly immunization of the animal, blood collection is carried to measure the amount of the monoclonal antibody using techniques such as flow cytometry and ELISA. This can give the idea of when the animal should be euthanized. After certain level of the antibody is reached, the animal can be euthanized for spleen, which can be used for small scale antibody production.
Fusion between myeloma cells and B cells
Prior to fusion, it is important to prepare the myeloma cells, which are prepared by culturing with 8 – azaguanine. Therefore, making them to be sensitive to hypoxanthine-aminopterinthymidin (HAT) medium. Then, the spleen cells producing the antibodies are fused with myeloma cells through the action of membrane fusing agent called polyethylene glycol [64].
Selection of the right fusion
It is always not all myeloma cells that will fused with the B cells. After the fusion, there will be fused myeloma cells with B cells, unfused myeloma cells, unfused B cell, B cells fused to B cells, myeloma cells fused to myeloma cells. To select only the myeloma fused B cells, the cells are grown in hypoxanthine, aminopterin and thymidine (HAT) medium, the hypoxanthine and thymidine are metabolite for salvage pathway of nucleotide synthesis, thymidine block the de novo pathway of nucleotide synthesis. The myeloma cells are HGPRT negative, therefore, only the B cells that possessed the HGPRT salvage pathway will be able to survive. Both cells contain de novo synthesis pathway. However, aminopterin in the medium will block the salvage pathway in both cells, only cells that are HGPRT positive can survive. Since the B cells are HGPRT positive, they can survive even if the de novo pathway is blocked, but myeloma cells cannot survive in HAT medium because they are negative of salvage pathway and the media component will block the de novo pathway. But if the myeloma cells fused with the B cells, they would compensate for the lack of salvage pathway i.e. myeloma cells would survive using the B cell salvage pathway, this is possible only if they fused together. Even though B cells can survive in HAT medium, they have limited life span, myeloma compensate for the shorter life span of the B cells. Hence, myeloma cells help the B cells to grow and differentiate [65].
Limited dilution for isolation of monoclonal antibodies of single specificity
After fusion and HAT selection, there is presence of antibody of different specificity, to separate them, the cells are then distributed into 96 wells, in which the walls of the wells are coated with either murine bone marrow macrophage or feeder cell from mice. The coating of the walls provide growth promoting factors to the cells producing the antibody (Figure 9A) [66].
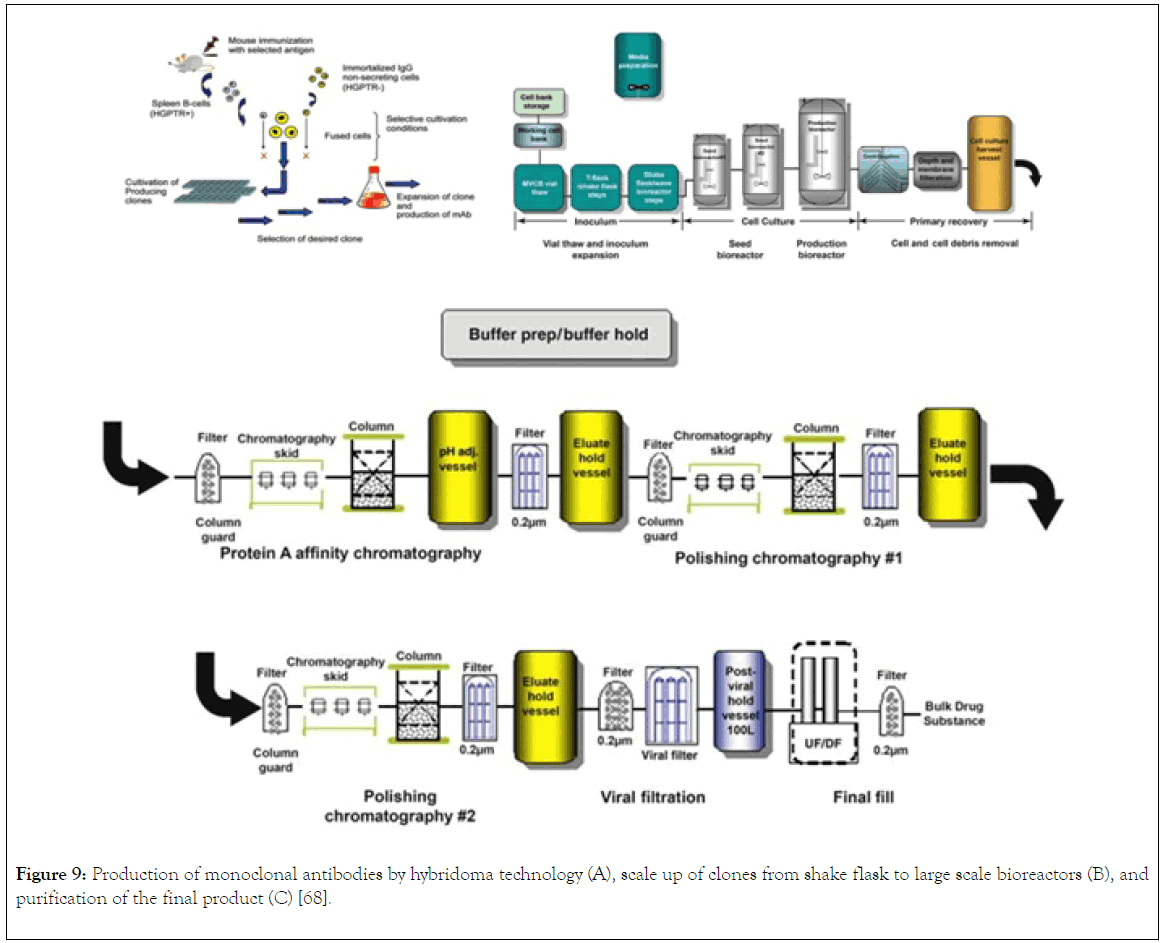
Figure 9. Production of monoclonal antibodies by hybridoma technology (A), scale up of clones from shake flask to large scale bioreactors (B), and purification of the final product (C) [68].
Screening for the target monoclonal antibody
To identify the right antibody, techniques such as ELISA and radio immuno assay (RIA) can be used. For the commercial production, the desired antibody from a sing well can harvested and used as cell bank for batch cultivation of the cells for the large-scale production of the specific antibodies.
In this instance, the hybridoma are grown in batch or fed batch bioreactor (Figure 9B). using fetal bovine serum as the media component. However, the used of bovine is associated with draw back like contamination with bovine immunoglobulin. Alternatively, the use of serum free for commercial production has been developed by many companies. The described method is associated with low yield of monoclonal antibody, strategy to increase yield include spinner flasks and roller bottles that keep the culture medium in constant circulation and thus permit nutrients and gases to distribute more evenly in large volumes of cell-culture medium.
Commercial production of currently registered mAbs is based on the cultivation of cell banks mammalian cells generated from generated from hybridoma. Industrial production of mAbs comprises of complex steps, in which each step contributes to the quality of the drug. Therefore, the general process for the production of mAb involves the use of genetic engineering and recombinant DNA technology to isolate gene (mRNA) coding for heavy and light chain from murine hybridoma cells generation. The strategy for the generation of murine cells hybridoma was discussed under small scale production of monoclonal antibodies. From the mRNA isolated, cDNA is synthesized using reverse transcriptase enzyme. Then, PCR amplification of light and heavy chain using primers specific for light and heavy chain. Cloning of light and heavy chain DNA into separate vectors [18]. Transformation of the vectors into mammalian cell line and selection of the cells producing the desired monoclonal antibody. After isolation of the most suitable clone based on product characterisation and titre, cryopreservation of the clone as cell banking for scale up to bioreactors for large scale production Fig. The mammalian cell line used for the production of mAb with the exception of the two mAbs produced by hybridoma technology are NS0 and SP2/0 cell lines or Chinese hamster ovary (CHO cell line) origin (Figure 9A). Example of monoclonal antibody produced by murine hybridoma are Othocone and Bexaae, CHO are Avastin, Rituxan, Herceptin and Humar, NS0 are Mylotag, Zenapx, and Synagis, and Sp20 are remicade and Erbutex. CHO are originated from Chinese hamster ovary which are proline auxotroph and diefient in dehydrotetrafolate enzyme which allows for the selection in the presence of metotrexate as inhibitor. The plasmid can then be design to contain a gene for DFRE enzyme, it is only the cells that stably integrate and expressed the DFRE gene that will be able to survive in the presence of the inhibitor. NS0 and SP2/0 are originated from mouse plastomacytoma cells that have been genetically engineered to yield non-IgG B cells that are immortal. These cell lines are used extensively in production of therapeutics monoclonal antibody with amenability to scale up to large scale fermenters. The downstream processing of monoclonal antibody after production in Bioreactors involve serial of steps (Figure 9B and C) [67]. The purification process for the isolation of monoclonal antibody is by harvesting the cell cultured fluid from bioreactor using industrial continuous disc stack centrifuges, then clarification using depth and membrane filters. Followed by capturing the mAb using protein A Chromatography, low pH elution step is included which is also a means of viral inactivation. In order to meet the satisfied and specified standard, two additional chromatographic polishing were incorporated in the downstream processing, which are anionic and cationic exchange chromatography. Also, a virus retentive filtration step is essential in ensuring that the final product is safe from. Concentration and formulation are the final step in the downstream processing, this is important in ensuring the quality, half-life, and of the drug [68]. The monoclonal antibody concentrated can be stored frozen or in liquid form and then transported to the drug manufacturing site [68,69].
It can be said that epitope binding site located in the variable region, antibody binding site located in the variable domain, Fc glycosylated region, hinge region that link the two heavy chains, all play an important role in antigen binding, clearance, antibody stability, receptor binding and immunity mediation. Hence, IgG 1 is the most commonly used as therapeutics because of its stability, half-life, less aggregation formation, higher serum concentration, IgG 2 is also use for therapeutics but much less than IgG1 and 4 because it is less stable than IgG 1, which is accounted by the disulphide bond in the hinge region but it possessed longer half-life, higher serum concentration than IgG 4 and 2, placental crossing, and complement activation. IgG 4 is also utilized for therapeutics which is due to it stability less aggregation formation, longer half-life and placental crossing even though it concentration is low in the serum unable to activate complement pathway. However, IgG 3 is not utilized for therapeutics due to its high chances of aggregation and instability accounted by the hinge region and rapid clearance from the serum. Hence structure forms an important role in IgG. Advanced in genetic engineering will enable IgG3 to be explore for therapeutic development.
Citation: Muhammed Y (2020) The Best IgG Subclass for the Development of Therapeutics Monoclonal Antibodies Drugs and their Commercial Production: A Review. Immunome Res. 16:173. Doi: 10.35248/1745-7580.20.16.173
Received: 12-Feb-2020 Accepted: 20-Feb-2020 Published: 07-Mar-2020 , DOI: 10.35248/1745-7580.20.16.173
Copyright: © 2020 Muhammed Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : none