Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Review Article - (2022)Volume 18, Issue 2
Immune Checkpoint Inhibitor (ICI) therapy is the most exciting development in cancer treatments in recent years. As of the end of 2021, more than a hundred of clinical trials have been carried out by several major drug makers and research hospitals all over the world just to explore the use of ICI antibodies in almost every type of cancer and under various clinical settings. These clinical trials have resulted waves of good news in every year’s major academic conferences and FDA approvals, and now ICI therapy is in the move to take over traditional therapy to become the major player in clinical management of cancer. On the other hand, most of these clinical trials have failed to show clear benefits in most cancer patients, and only few have been reported in public. In real-world clinical setting, ICI antibodies are being massively and sometimes abusively prescribed for almost every desperate situation with clear clinical benefit in only a few (<10%). Yet, the enthusiasm behind ICI therapy is not dampened by the massive clinical failure but has been growing. The reason for the consistent enthusiasm among clinicians and patients is the miraclelike clinical responses seen in some of the responders that even including late-stage hopeless cases. Every clinician wants to repeat this miracle in the next seemingly identical patient, and every patient and their family members want to believe that they are in line for that miracle. But the reality is disheartening in that clinicians do not see the predictable repeat of miracle-like responses in seemingly identical patients and most patients selecting ICI therapy as the last straw in life did not benefit from it, if not hurt by it. What then are the reasons that ICI therapy is so impressive when working and so unpredictable in responses? This review attempts to draw some mechanistic aspects based on our own experiences in ICI therapy and to come up with a different explanation for the unexplained clinical observations. The goal of this review is to alert the clinical field about the danger and irreversible damages that wrongly used ICI antibodies may cause, and at the same time to introduce some preliminary criteria to select proper patient for the benefits and to avoid the harms of ICI therapy.
ICI therapy; Immune checkpoint inhibitor; Depletion model
Real-world use and responses of ICI therapy
ICI therapy is composed mainly by the use of antibodies to the Progressed Death Receptor-1 (PD-1), its ligand PD-L1 and another cell surface checkpoint receptor CTLA-4. As of 2021, there are at least 13 antibodies against PD-1/PD-L1 and one against CTLA-4 have been approved for sale on the market.
Among them there are 8 antibodies to PD-1, occupying most market sales, reaching over $30 billion in 2021. With this trend, one of these antibodies, pembrolizumab (Keytruda) have overtaken the number 1 position for cancer drug in the world. On the other hand, approved uses of ICI antibodies rarely apply to front line single-use setting, mostly are associated with chemotherapy under the conditions of failed previous treatments. Some of the approved uses are so rare that only very few patients actually qualify for the condition. This observation indicates that among large number of clinical trials, the benefits of ICI therapy are more likely to be visible in desperate, but not freshly diagnosed cases. This is not because ICI therapy performs better under that situation than if applied to earlystage cases. It is more likely that the comparison therapies for the control group are in general less effective in late stage and previously treated patient, leaving room for ICI responses to stand out. Had ICI therapy been really effective as over exaggerated on social media platforms, we would have stopped using other therapies by now. The fact that ICI therapy remains an accessary and mostly second line treatment option indicates that it is not highly effective, at least not good enough to bring clear clinical benefit to the majority of patients. On the other hand, at least in China where the largest cancer patient base exists and multiple domestic ICI antibody suppliers compete intensively, the off-label use of ICI therapy has become more and more a common play in cancer management. In our own observation, many patients were prescribed ICI therapy alone or combined with chemotherapy or other targeted drugs (such as tyrosine kinase inhibitors) without following the appropriate selection criteria by the approval agency, but were based on desperate needs to control the disease. In these cases, ICI therapy serves as the “last hope”. The accurate accounts of such massive and sometimes abusive uses of ICI therapy (see later discussion) may never be reported in publication, but it is a reality taking places in every day cancer clinics. One may want to know with such broad use in many cancer cases and the rapid jump year over year in drug’s sale, how many cancer patients have benefited from this practice? Are the published, well controlled clinical trial data replicable in real world use? The answers, at least based on our own experiences, unfortunately are more negative than what we have hoped to be. It is true that few patients have benefited tremendously from ICI therapy, some even been cured of their diseases. But many more did not benefit from the therapy. Some recent reviews of non-selected use of PD-1 antibodies in lung cancer, the largest approved usage category, revealed that the actual responder ratio may go as low as 12% [1] and real-world survival following ICI therapy is shorter, sometimes by a large difference [2,3]. In our own observation, off-labeled use of PD-1 antibodies in other solid tumors such as esophageal, stomach and liver cancer in China is reaching above 80% patients in some clinical centers. Yet, despite some patients showed dramatic reverse of their disease course and benefited from the therapy, most do not show clear benefits from ICI therapy. A tragic observation is that many more patients under such non-discriminate use of ICI therapy have actually been hurt by it.
Rarely discussed among clinicians and mistaken by most patients and their family members, may ICI cause disease acceleration in various forms? The most obvious form is a short time burst of tumor progression in both sizes and numbers, leaving a clear impression that ICI therapy is the cause of the hyper-progression [4]. But many times, it is not so obvious in that disease progression took places after several dosing of ICI antibodies, leaving the impression that the therapy simply did not work. In many times, this interpretation is wrong because careful observation may reveal that the therapy did bring positive responses initially (at least by the indication of tumor markers). It was the continued dosing of ICI antibodies that caused the subsequent loss of efficacy. In most cases, it is not a simple loss of previous responses but a beginning of accelerated progression for that patient. As later discussion of mechanism may show this is due to the loss of antitumor immunity. Based on our own experience with ICI therapy in the past 5 years (to be reported elsewhere), the pattern of responses to ICI therapy is likely to be divided into three categories of responders, nonresponders and victims, with each portion shown in (Figure 1). Among all users of ICI therapy, true responders occupy about no more than 25%. At the same time, about 40% of users are victims, leaving the rest 1/3 patients as true non-responders. It needs to be point out that we have been aware of the hyperprogression very early on and thus we carefully selected patients whose tumor showed clear sign of PD-L1 expression by our comprehensive evaluation methods that include observation of tumor tissue and following tumor marker changes, and we always used delayed dosing schedule to avoid damage to antitumor immunity. It is alerting that even under such scrutinizing condition, we have obtained more ICI therapyinduced harms than benefits. Our estimation for the situation in the general field should only be worse. The true benefiters may account as low as <10% patients and the victims of the therapy may account more than 50%. The rest should be nonresponders due to lack of systemic T cell depletion (see next section) and lack of pre-existing antitumor immunity to be activated by ICI therapy. In our hands, we carefully selected patients with adequate concomitant antitumor immunity and excluded patients whose tumor had no concomitant antitumor immunity, thus to lower the non-responder portion. The striking issue to be considered here is the awareness of existence of victims other than simply non responders of the ICI therapy in the general field out there (Figure 1).
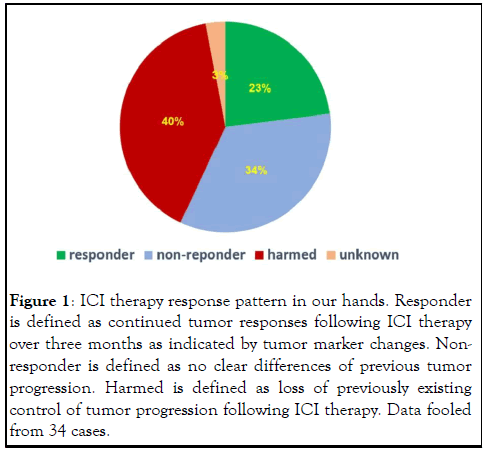
Figure 1: ICI therapy response pattern in our hands. Responder is defined as continued tumor responses following ICI therapy over three months as indicated by tumor marker changes. Nonresponder is defined as no clear differences of previous tumor progression. Harmed is defined as loss of previously existing control of tumor progression following ICI therapy. Data fooled from 34 cases.
Challenging clinical observations to the proposed working model of ICI therapy
In light of some of the perplexing clinical observations from ICI therapy, the current explanation on how ICI antibodies reach antitumor effect is rather weak or even self-defeating. Based on the model adopted by the mainstream medicine, ICI antibodies block the interaction between PD-1 and its ligand PD-L1, thus blocking the down regulation of PD-L1 through the interaction with PD-1 on activated T cells. PD-L1 could is on tumor cells or on other immune accessary cells (such as dendritic cells or macrophages), regardless, its interaction with PD-1 on activated T cells causes down regulation of T cell activation and thus antitumor activity. We refer to this mechanism as the “blocking model”. According to the Nobel Prize Committee, 2018 Nobel Prize for Physiology or Medicine was awarded to two scientists who worked on immune checkpoint mechanism for “their discovery of cancer therapy by inhibition of negation. With the Nobel Prize Committee standing behind, there should not be any doubt about the mechanism of ICI antibodies in cancer therapy as the blocking model depicts. Yet, there is no direct evidence that the antibodies work in vivo by this mechanism. For example, despite the model assumes that antitumor immunity is inhibited by tumor expression of PD-L1, there is no study showing this is actually happening in vivo inside tumor. In the contrary, study including our own indicate that PD-L1 expression is the result of strong immune attack on tumor through IFN production at the site of tumor [5,6]. Despite many in vitro studies showing PD-L1 induces T cell death (thus the name programmed cell death receptor), there is no in vivo study showing that tumor expressed PD-L1 could cause T cell death or down regulate T cells activation at the site of tumor. Instead, our own unpublished observations in tumor sections always show large number and highly activated T cells co-exist with PD-L1- expressing tumors (Figure 2). It is true that these activated T cells may not suppress tumor replication anymore, not only is tumor replication not suppressed upon expressing PD-L1, it usually increases (Figure 3), form a clear stalemate between tumor and antitumor immunity (Figure 4). But this standoff is something taking place inside the tumor cells, not the result of suppression of T cell activation because there is no establishment of new metastasis under such standoff, indicating that T cells unable to suppress existing PD-L1-expressing tumors are capable to destroying newly formed tumors that do not have opportunity to express PD-L1 before being destroyed. Even with the standoff, there is no study showing that tumor-confronting T cells such as those in (Figure 5) resume attack on tumor cells after bond to ICI antibodies. In addition, this blocking model cannot explain several common observations from the actual use of ICI antibody. These observations are as follows (Figures 6,7).
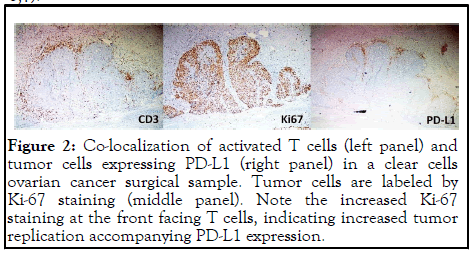
Figure 2: Co-localization of activated T cells (left panel) and tumor cells expressing PD-L1 (right panel) in a clear cells ovarian cancer surgical sample. Tumor cells are labeled by Ki-67 staining (middle panel). Note the increased Ki-67 staining at the front facing T cells, indicating increased tumor replication accompanying PD-L1 expression.
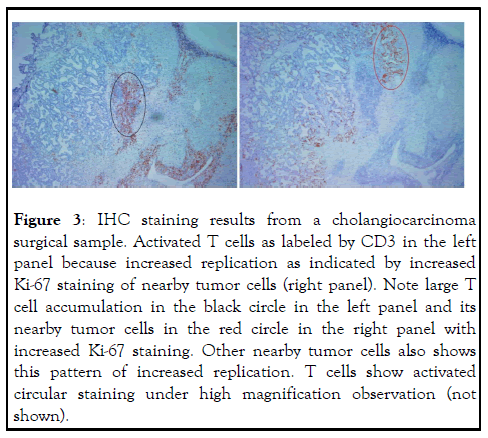
Figure 3: IHC staining results from a cholangiocarcinoma surgical sample. Activated T cells as labeled by CD3 in the left panel because increased replication as indicated by increased Ki-67 staining of nearby tumor cells (right panel). Note large T cell accumulation in the black circle in the left panel and its nearby tumor cells in the red circle in the right panel with increased Ki-67 staining. Other nearby tumor cells also shows this pattern of increased replication. T cells show activated circular staining under high magnification observation (not shown).
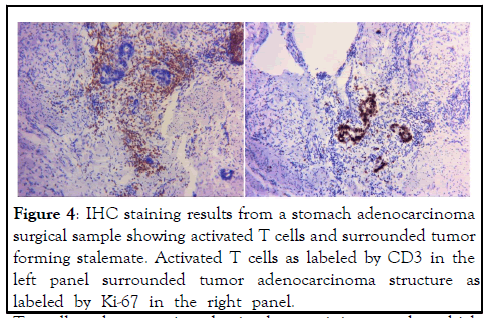
Figure 4: IHC staining results from a stomach adenocarcinoma surgical sample showing activated T cells and surrounded tumor forming stalemate. Activated T cells as labeled by CD3 in the left panel surrounded tumor adenocarcinoma structure as labeled by Ki-67 in the right panel.
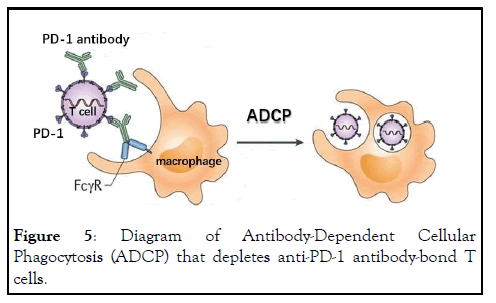
Figure 5: Diagram of Antibody-Dependent Cellular Phagocytosis (ADCP) that depletes anti-PD-1 antibody-bond T cells.
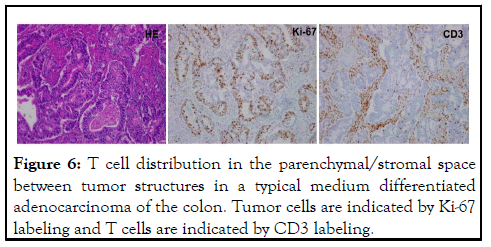
Figure 6: T cell distribution in the parenchymal/stromal space between tumor structures in a typical medium differentiated adenocarcinoma of the colon. Tumor cells are indicated by Ki-67 labeling and T cells are indicated by CD3 labeling.
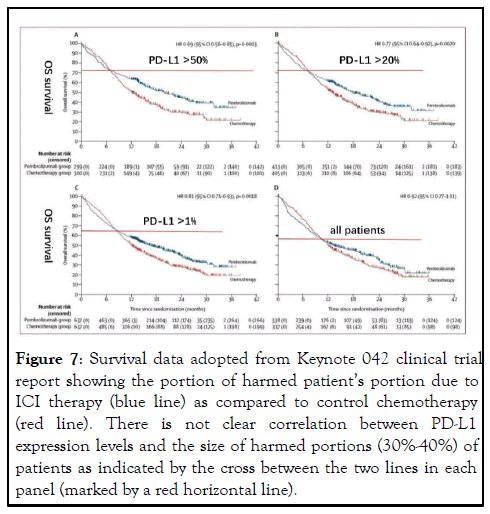
Figure 7: Survival data adopted from Keynote 042 clinical trial report showing the portion of harmed patientâ??s portion due to ICI therapy (blue line) as compared to control chemotherapy (red line). There is not clear correlation between PD-L1 expression levels and the size of harmed portions (30%-40%) of patients as indicated by the cross between the two lines in each panel (marked by a red horizontal line).
T cells show activated circular staining under high magnification observation (not shown). Despite this strong T cell attack, tumor structure remained intact and tumor replication apparently increased as indicated by stronger Ki-67 staining compared to tumor staining pattern in other area of less or no T cell presence (not shown).
The “trigger” effect often associated with dramatic and durable responses. Although drug makers recommend repeated dosing of ICI antibodies based on the blocking model and the pharmacokinetics of antibody in vivo, it is now commonly known that durable and persistent responses are seen in patients even after stop of continued ICI antibody [7-10]. In our own experience, we have seen a single dose of ICI antibody triggered a dramatic antitumor response lasting over 10 months. This phenomenon cannot be explained by the blocking model which requires presence of ICI antibodies throughout the entire action period. It is well known that cell surface formed antigenantibody complex is constantly internalized in the matter of hours and despite the claim that ICI antibodies have in vivo halflife of 2-3 weeks, the pharmacokinetics of these antibodies do not match their dose-response profile [11], indicating that it is not that more antibody presence brings more responses (Figure 7).
Hyper-progression after ICI therapy, All along the clinical trials, a phenomenon called “hyper-progression” has been brought up occasionally to describe a group of patients whose tumor growth dramatically accelerated after ICI therapy [12]. Definition of hyper-progression has been changing but the essential features of hyper-progression are that tumor progression increased in speed and new nodules developed. The reported portion of hyper-progression varied among studies and reports, ranging from as 4%-40% [13]. We have adopted a broader definition of ICI therapy-caused acceleration of tumor progression by looking both changes of tumor markers that reflect acceleration in trend and tumor distribution that show new establishment of metastases. Instead of using the term “hyper-progression” that only focused on a small portion of victims whose tumor “exploded” after ICI therapy, we use the term “harm” to include all patients who are hurt rather than helped by ICI therapy. This is because the mechanism behind the damage is the same among these patients (see next section), only differing in the degree of damages, a factor that is also heavily influenced by the biological behavior of each individual tumor. In our own hands, as Figure 1 shows, this portion of patients accounted up to 40%. Thus, it is not a small portion of patients that may be hurt by ICI therapy, but rather a major portion, at least more than benefiters. From the view of tumor immunology, hyperprogression is the net result of loss but not enhancement of antitumor immunity. How can a therapy that intended to activate or strengthen antitumor immunity result in destroying it? There have been some studies attempting to provide a mechanism behind hyper-progression, but regardless whether these findings or hypotheses are relevant; this phenomenon cannot be explained by the blocking model [14-16].
ICI therapy caused autoimmunity. May be not a larger portion, but some patients experience autoimmune attack following ICI therapy. This portion of patients may account more than 10% in some trials. In our own hands, we saw >10% of patients experiencing some forms of autoimmunity following ICI antibody dosing. These autoimmune attacks are not short-lived and last as long as the durable tumor responses in some patients. In two isolated cases the autoimmune attacks in the lung were serious enough to have caused patient death despite persisted suppression of immune attack by corticosteroids. There is no correlation between autoimmunity and antitumor efficacy, but we have not seen a case in which a non-responder experienced clear autoimmunity: i.e., in every case where autoimmunity was observed, the patient either benefited or was hurt by the therapy. Then how is the autoimmunity before ICI therapy prevented and how is this prevention broken by ICI therapy and how do we know that the same protective mechanism is not used by tumor? Extensive studies in the field of autoimmunity have long established the mechanism of peripheral tolerance and the role of regulatory T cells (Treg) as the main player for prevention of autoimmunity. The main mechanism is through suppressive cytokine to inhibit the activation of effector cells (Teff), although the role of PD-1 and PD-L1 has been implicated in several autoimmune models [17]. In all cases, PD-L1 expression of the target tissues seems necessary, indicating a parallel and additional protective mechanism to the Treg-mediated suppression in autoimmunity. From immunological point of view, autoimmunity activation by ICI therapy should share the same mechanism of antitumor immunity activation. But one can hardly imagine this autoimmunity was blocked by PD-1/PD-L1 interaction before ICI therapy like what is proposed for antitumor immunity. At lease target tissues of autoimmunity such as normal lung does not express PD-L1 in order not to be attacked by autoimmunity.
In addition, as mentioned above, autoimmunity following ICI therapy often lasted long and resisted even severe immune inhibition, which also rules out the involvement of PD-1/PD-L1 in maintaining suppression of autoimmunity, and therefore cannot be explained by the blocking model. We bring this issue up not to focus on the presence of immune-related adverse events of ICI therapy, but to emphasize the mechanism behind. After all, it is the activation of a previously suppressed immune response, the same as antitumor immunity.
New mechanism for ICI therapy and supporting evidences
From lack of direct proof of the proposed blocking model to the failure to explain these above listed clinical observations by the blocking model in a decade-long period and under extremely high field enthusiasm, one has to raise doubt about the correctness of the blocking model. The fact that these issues, thorny as they are, remain unsolved must indicate that the blocking model is likely wrong. Then the question is what is a correct mechanism of ICI therapy and how can we explain all of the above observations? Here we propose a new mechanism for ICI therapy that covers all of the clinical observations mentioned above. The essential aspect of this proposed mechanism is antibody-mediated T cell depletion. This depletion is most likely through Antibody-Dependent Cellular Phagocytosis (ADCP) in which ICI antibody-bond T cells are removed by macrophages (Figure 5). ADCP is a well-recognized process in vivo that removes antibody-bond objects that plays critical role in pathogen control. Macrophage recognizes the Fc fragment of the antibody through its cell surface Fc Receptor (FcR) and the binding results in the engulfing of the antibodyantigen complex [18]. Inasmuch as all ICI antibodies contain Fc segment that can be recognized by macrophage, this removal of ICI antibody-bond T cell is a possibility and potentially a problem. In fact, it is a common and highly efficient practice to deplete T cells in vivo through this mechanism in immunology research in animal models (for example, depleting T cells with anti-CD4/CD8 antibodies). Although ICI antibody developers claim that they are aware of this potential problem, and have thus modified the Fc region in their products to reduce the degree of FcR binding, there is no evidence that these ICI antibodies lose the ability to be recognized by macrophages in vivo and antibody-bond ICI antibodies are not removed in vivo. In fact, there is study showing binding of ICI antibodies through Fc segment and depletion of antibody-bond T cells in vivo [19]. This T cell depletion mechanism may well explain the damage caused by ICI therapy in that antitumor T cells that express PD-1 is bond and removed through ADCP process. When these T cells are critical for suppressing tumor development, their removal, partial or complete, would therefore result in tumor progression in various degrees, not necessarily to be hyper-progression in every case because establishment of new metastasis is also controlled by tumor and its interaction with the host microenvironment. By the same mechanism, the autoimmunity activated by ICI antibodies is also the result of T cell depletion, only the target T cells that bond antibody and removed by ADCP are Tregs that carry out peripheral tolerance and maintain suppression of autoimmunity. This would result in the release of previously suppressed T effector cells that carry out the immune attack. If these effector T cells also express PD-1, they would also be removed by subsequent dosing of the same antibody that caused the depletion of Tregs. But if the re-activated autoimmune T cells do not express PD-1, the subsequent dosing of ICI antibodies would not affect the immune attack. Removal of Treg could also be the mechanism in some patients where the antitumor immunity is suppressed by Treg. However, this is not the main mechanism because higher responses are seen in patients whose tumor express PD-L1, and PD-L1 expression is the hallmark of Th1 immune attack [20], not an indicator of Treg. Only in cases where PD-L1 expression was not detected, ICI-activated antitumor immunity could be through the removal of Treg. Same as in the case of autoimmunity, continued administration of ICI antibody may cause the removal reactivated antitumor T cells, thus neutralizing the initial activation, leaving highly variable results, which is most likely the real situation in the clinical setting. The most difficult-toexplain question by this T cell depletion model is how antitumor responses are activated. As discussed above, if such T cells are completely removed, there is a loss of antitumor immunity resulting in tumor progression/hyper-progression. Thus, in order for the depletion model to be feasible one has to assume the following :1) T cell depletion must take place because the entire model is built on removal of T cells expressing PD-1; 2) T cell depletion cannot be complete, otherwise resulting in loss of tumor control; 3) Unresolved T cells must be activated through this process. The partial removal of T cells is thus the key in this depletion model. T cells may not be removed if they do not express PD-1, or they are not bound by antibody. The latter is possible since tumor microenvironment is complex and it is known that many drugs especially large molecules such as antibodies cannot easily penetrate everywhere inside a tumor. It is thus not surprising that some T cells, especially those that deeply infiltrate into the tumor are spared by the antibody in circulation. This alone cannot explain T cell activation, but leaves one opportunity for remaining un-bond T cells to be activated if they replace depleted T cells thorough homeostatic process. This proposed model assumes that this homeostasis results in temporary T cell activation and expansion. In the case where this expansion takes place rapidly within days of initial T cell depletion, one may expect to see a net increase of antitumor T cell that have the most antitumor activities (because they are the ones that are able to penetrate into the tumor, a highly desirable feature for antitumor immunity). In this model, we do not have to assume that the T cells not removed by ICI antibody do not express PD-1, but observations discussed below indicate that there must be some activated T cells that do not express PD-1, and there are also some T cells that do express PD-1 and are subject to subsequent antibody-mediated depletion. The actual status of PD-1 expression following ICI therapy has been shown to be mixed [21], but either way, it does not affect T cell activation following partial T cell depletion.
By proposing this T cell depletion model, we are able to explain the above listed clinical observations that cannot not be explained by the on-going blocking model. Furthermore, some clinical observations and animal study results support this model. First, this model predicts that ADCP process is critical for ICI antibody to work in vivo, thus antibody with binding and blocking function but lost the ability to go through ADCP would not work. Indeed, Fab fragments of ICI antibody have been looked but not reported to work, despite the desirable features of such antibody to penetrate tumor tissue better in vivo. Even antibody with intact Fc segment but engineered to specifically disrupt the Fc receptor binding site showed loss of antitumor activity in a mouse tumor model in vivo [22]. Secondly, unlike the blocking model that predicts equal chance for antibodies to either side, this depletion model emphasizes on depletion of T cells, not tumor. Between antibodies recognize PD-1 and PD-L1, the former would be more relevant than the latter since PD-1 is mainly expressed on T cells although some analyses also indicate that PD-L1 is also expressed by activated T cells. From this comparison, one expects that anti-PD-1 antibody would be more active than anti-PD-L1 in vivo. Thus far, this prediction is generally supported by today’s clinical observation and drug sale numbers. Even among the lagging activities of anti-PD-L1, some may be contributed by the binding of this antibody to T cells, but not tumor. We have seen at least one case clearly supporting this explanation (our unpublished observation). Thirdly, this model depicts that the degree of T cell depletion is critical in that over depletion would lead to the loss of antitumor immunity and control of tumor progression. Only “proper” partial depletion leads to replacement of depleted T cells through homeostasis recovery and T cell activation. T cells located in the stromal/parenchymal space between tumorformed structure such as all medium and high differentiated adenocarcinoma tumors are more accessible to antibody and are more prone to depletion. In support of this view, all hyperprogression cases in our hands fit this picture. ICI therapy do not work well in tumors with typical medium-to-high differentiation structure, such as most colon cancer, some lung cancer and most ovarian cancer cases as compared to low differentiated tumors such as melanoma. Figure 6 is an example what a medium-differentiated tumor looks like. It is clearly different from the tumors shown to respond well to ICI therapy [23-26]. One exception would be in the case of Treg suppressed antitumor immunity in which Tregs located in the stromal space are likely depleted by ICI antibodies. We have seen one such case in our hands. Fourthly, this depletion model requires one to witness T cell depletion following antibody administration. In animal models, such depletion is rapid within one day, and in patients, we expect to see clear drop of lymphocytes within 2 days. Indeed, in our hands, we saw drop of lymphocytes to various degree ranging from 12%-54% by 50 hours following ICI antibodies. Severe drop (>30%) of lymphocyte accompany either responses or damage, and minimal drop (12%-15%) is often an indication of no response nor damage (our unpublished observations). Finally, our depletion model also explains the observation of temporary responses or progression (pseudo-progression) in ICI therapy. Due to T cell depletion, it is possible that some antitumor activities are lost after initial antibody administration. While this partial depletion also leads to T cell activation, subsequent catch-up by the activated immunity finally controls the progressing tumor and brings about responses. During this time, tumor progression may actually accelerate before subside. This temporary increase of tumor progression has been called pseudo-progression based on radiographic imaging tests and have been explained as immunity-induced swollen by immune cells into the tumor. We know that the temporary tumor progression is a true but not pseudo-progression based not just on imaging, but also on tumor marker changes. On the other hand, quite a large portion of cases have shown initial responses and relapses following continued antibody administration. We suspect that this shortlived response is caused by accumulated partial depletion leading to net reduction of antitumor T cells too much to sustain antitumor responses. With this homeostasis-driven T cell activation, T cell infiltrating deeply inside tumor may need to exit to parenchymal/stromal space and even draining lymph nodes for expansion and if the expanded T cells express PD-1, they are prone to depletion by continued antibody dosing. It may all depend on the location and distribution of T cells when the depleting antibodies are in the circulation. Based on our observation of various modes of T cell infiltration, in majority cases with concomitant antitumor immunity, T cells are in the stromal/parenchymal space like Figure 6 shows, thus theoretically not possible to respond to ICI therapy in the current form and frequency. Patients in these cases will be hurt due to over depletion of their concomitant antitumor immunity if forced to accept ICI therapy in the current form.
One puzzling issue to be discussed is the dramatic and durable responses seen in a minority of ICI responders. The antitumor response in these patients is so strong that it lasts in months that no previous cancer therapy can match. According to our understanding of the activation process of an immune response, especially antitumor response, antigen release is absolutely necessary to initiate and maintain the activated immune response. But one confounding puzzle in the durable antitumor response activated by ICI therapy is the obvious lack of antigen release that triggers the activation. Inasmuch as PD-1 binding by antibody does not trigger T cell activation and does not kill tumor cells (something easily testable in vitro), the mode of T cell activation following ICI antibody remain unclear. We try to explain this special way of T cell activation by homeostasisdriven compensation process following ICI antibody-mediated partial T cell depletion, but it remains a weak point of this model since homeostasis-driven T cell recovery is common following other tumor therapy such as chemotherapy and no such activation has been observed. There must be something special about the environment following ICI antibody administration that leads to a selection of highly activated antitumor responses. One possible feature is that in the case of ICI antibody-mediated depletion, the depleted T cells all express PD-1, thus only PD-1 negative T cells may survive the postdepletion recovery since there are still some antibody molecules remaining in the circulation for weeks. Durable responses are likely sustained by these PD-1 negative but activated T cells. Such cells do exist as previous study indicated that highly activated T cells modified by third activation signal such as IL-12 would resist all forms of down regulation by shedding PD-1 expression [27-29]. In support of this view, our previous antitumor studies with IL-12 did observe this kind of “selfsustaining” responses [30]. In addition, following activation and during the long-lasting response it is not clear how this response is maintained without clear mode of antigen release. Tumor antigens are released by tumor-reductive therapies such as chemo and radiation therapy that kill tumor cells. But in the case of ICI therapy, it is not necessary to rely on other tumor-reductive therapies to be present (although recent applications often include chemotherapy), thus ruling out the classic mode of tumor antigen release. On the other hand, antitumor responses activated by antigen release such as after chemotherapy is not long-lasting and requires repeated treatments [31]. This is clearly the case in cancer clinics as repeated chemotherapy is needed to suppress tumor progression. Then, in clear contrast and comparison, one needs to ask how is ICI therapy-activated antitumor response sustained? As the need for antigen cannot be bypassed for that would violate the specificity aspect of response, one has to assume that the ICI therapy-activated immunity is different from what we see in other cancer therapyactivated antitumor response in that it is “self-sustaining” in antigen supply. Self-enhancing immune response has been proposed before but not supported by mechanistic studies, especially in tumor immunology field. On the other hand, ICI therapy-activated antitumor responses are not always selfsustaining, but only in minority cases. What then decides the difference is unclear?. Understanding the mechanism behind will likely to bring more self-sustained durable responses to more patients.
The prognostic significance of the new model
The new depletion model is not just for the explanation of why ICI therapy has shown dramatic and durable responses, although it does help to understand the true mechanism behind. Because of the potential harm ICI therapy may bring to a large portion of patients, it becomes desperate to establish new patient selection criteria that could avoid this problem. Current patient selection criteria clearly cannot do so because previous clinical trials did show a large portion of patients, ranging from 30%-40% actually had shorter survival than the control group [32]. For example, selection of patients whose tumor expresses high levels of PD-L1 has been used initially to show that more responders were in this group. However, no significant reduction of harm was obtained in that there was still larger portion of patients survived shorter than controls (Figure 7). In our hands, all patients subjected to ICI therapy had clear sign of tumor expression of PD-L1 under strong immune attack, but still 40% of them were harmed rather than helped by the therapy (Figure 1). Other selection criteria such as TMB, MSI and dMMR status were also proposed and adopted by FDA in their approval, but none has the ability to avoid ICI induced harm to immunity. These surrogate markers all point to the likelihood of presence of concomitant antitumor immunity.
Although this is necessary as a prerequisite for ICI therapy, the presence of this immunity alone cannot avoid being depleted by ICI antibodies and thus being hurt by the therapy. The depletion model as depicted here would have the ability to identify patients who may be hurt than helped by ICI therapy.
For each patient, the most critical things to look for are that 1) whether there is a presence of concomitant immunity; and 2) whether T cells are fully exposed in the stromal space that easily accessible to circulating antibody. All patients who meet these two observations (a majority in solid tumors) would be highly likely hurt and should be prohibited from entering ICI therapy. By this practice, we have thus far avoided all damage used to take place with ICI therapy except in one case where the patient had a mixed tumor of hepatocellular and cholangiocarcinoma cancer. In that case, the T cell distribution pattern was at a borderline situation for the major component (hepatocellular) and the minor cholangiocarcinoma tumor had very low presence of antitumor immunity that is too weak to be assessed. Treatment brought initial responses followed by subsequent hyper-progression following the 2nd antibody given after witnessing responses 4 weeks later (our unpublished results). On the other hand, since the adoption of this new model and its depicted patient selection criteria since June 2021, we have since recommended ICI therapy to 8 patients with all showed clear initial responses. Seven of them showed continued responses and one showed subsequent hyper-progression as discussed above. Inasmuch as non-selected use of ICI therapy in the field may not obtain more that 20% of responses and larger portion of damage to the patients, our record so far stands out with extremely high statistical significance (p<0.01), indicating that we must have something right in our hands. In a way, this is not the familiar way of proving a model since mainstream medicine is used to see randomized and controlled comparison between groups of patients for statistical significance. But to prove the depletion model as we have down in the past few months, there is also two principles that apply here: one is the ethics concern that we cannot select patients to accept ICI therapy as the current form as we know that they have very high chance to be hurt then helped. Two, inasmuch as we just need to prove that we are right with statistical significance, the zero-knowledge proof that surpasses explanation to experts who may not understand or do not believe this new model seems suffice. We have actually done that, only without monitoring referee.
We are willing to challenge the mainstream medicine to have their cases predicted by our model before subjecting their patients to ICI therapy. For drug makers, it is difficult to adopt our model, since the T cell depletion model clearly prohibits frequent dosing of antibodies due to over depletion of T cells and loss of concomitant immunity even under an initial response. Less use of ICI antibodies means less profit. The early involvement of the Nobel Prize Committee is also a strong barrier to the change of on-going blocking model. On the other hand, the reality of large number of hyper-progression cases associated with ICI therapy is a serious problem and a daily norm in cancer clinics that cannot go away by ignoring it. The addition of chemotherapy to ICI therapy only covers up short term explosion of tumor progression, but not solving the problem of antitumor T cell being over depleted. Scientists and clinicians sooner or later will realize this problem and find out the reason. This review, as an early exploration in theory and a late effort in correction after some deaths caused by ICI therapy in our hands, hopefully will bring alert and insight into this issue and serve as witness to the establishment of a new working model for ICI therapy. After all, ICI therapy has tremendous potential to bring significant benefits to about one-third of cancer patients, and we need to master its correct use to help, but not to hurt cancer patients.
Citation: Tsung K, Xu Z, Zhanghui (2022) The Blocking vs Depletion Model of Immune Checkpoint Inhibitor Therapy for Cancer. Immunome Res. 18:157.
Received: 02-Jun-2022, Manuscript No. IMR-22-17701; Editor assigned: 06-Jun-2022, Pre QC No. IMR-22-17701(PQ); Reviewed: 20-Jun-2022, QC No. IMR-22-17701; Revised: 02-Aug-2022, Manuscript No. IMR-22-17701(R); Published: 09-Aug-2022 , DOI: DOI: 10.35248/1745-7580.22.18.157
Copyright: © 2022 Tsung K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.