
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2020)Volume 10, Issue 1
Introduction: Flecainide is a class Ic antiarrhythmic agent used to prevent and treat both ventricular and supraventricular tachycardias including atrial fibrillation, AV nodal re-entrant tachycardia (AVNRT), and Wolff- Parkinson-White syndrome (WPW). Flecainide can cause serious side effects include cardiac arrest, arrhythmias, and heart failure. Despite its growing use, the presenting signs and symptoms of flecainide toxicity are not familiar to most clinicians.
Case description: This was a 58 year-old female who presented from her skilled nursing facility due to hypoxia and altered mental status. Past medical history was notable for paroxysmal atrial fibrillation for which she was on flecainide. At the nursing facility the patient was noted to be obtunded, whereas at baseline she was reportedly alert and oriented. On presentation to the Emergency Department EKG demonstrated wide-complex tachycardia. Subsequent lab results were notable for hypokalemia and an acute kidney injury (AKI). A repeat EKG was concerning for ischemia in leads II, III, aVF and cardiology was emergently consulted. They noted the EKG changes were consistent with flecainide toxicity, specifically a prolonged PR interval and QRS. She was eventually admitted to the medical intensive care unit. Her wide complex tachycardia on admission was ultimately attributed to flecainide toxicity in setting of AKI. Six days after presentation, it was found that her flecainide level was in the toxic range at 2.02 ug/mL (normal range: 0.20-1.00 ug/mL, toxic>1.50 ug/mL).
Discussion: Flecainide intoxication is rare but serious due to the potential for cardiogenic shock. Its diagnosis can be difficult as the flecainide serum level may take days to result and there are no pathognomonic clinical signs. This case demonstrates the necessity of keeping flecainide toxicity on the physician’s differential for patients who are taking the drug, as well as what ECG and laboratory findings can suggest it as the etiology. A heightened suspicion is warranted in the patient with new renal impairment. Treatment can be lifesaving if initiated promptly.
Flecainide; Antiarrhythmic agent; Tachycardia
Flecainide is a class Ic antiarrhythmic agent that blocks the sodium channel without having an effect on the action potential. It is used to prevent and treat both ventricular and supraventricular tachycardias including atrial fibrillation, AV nodal re-entrant tachycardia (AVNRT), and Wolff-Parkinson-White syndrome (WPW) [1-3]. The use of flecainide has grown over time with over one million prescriptions written in 2016.
Flecainide has a number of extracardiac side effects, most frequently dizziness and visual disturbances that often occur together. Other common adverse reactions include headache, nausea, dyspnea and chest pain [4]. More serious side effects include cardiac arrest, arrhythmias, and heart failure. As such, it remains a second line treatment in patients with serious arrhythmias or when significant symptoms cannot be managed with other agents [1]. In addition, it is not recommended in those with structural heart disease or ischemic heart disease [5].
This was a 58 year-old female who presented to the emergency department from her skilled nursing facility due to hypoxia and altered mental status. Her past medical history was notable for paroxysmal atrial fibrillation, Crohn's disease status post resection and colostomy, Type II diabetes mellitus, and idiopathic angioedema. Her medications included flecainide, apixaban, and prednisone. At the nursing facility the patient was noted to be obtunded, only wincing to painful stimuli, whereas at baseline she was reportedly alert, oriented, and able to communicate. The timing of her change in her status was unclear and was noted at shift change the morning of her presentation. An ambulance was called, and she was brought to the emergency department. En route, she was hypotensive with a blood pressure of 92/62. Soon after arrival to the emergency department she was intubated for airway protection. The cardiac monitor demonstrated a wide-complex tachycardia (Figure 1). Synchronized cardioversion at 200 J was delivered without change in her rhythm (Figure 2). No laboratory values had yet resulted and it was presumed that the EKG changes were due to hyperkalemia. Calcium gluconate, sodium bicarbonate, and insulin/dextrose were then administered, again with no change in the QRS duration. Subsequent lab results included a potassium of 3.0 with creatinine of 2.44 (baseline 0.8), and lactic acid of 3.9.
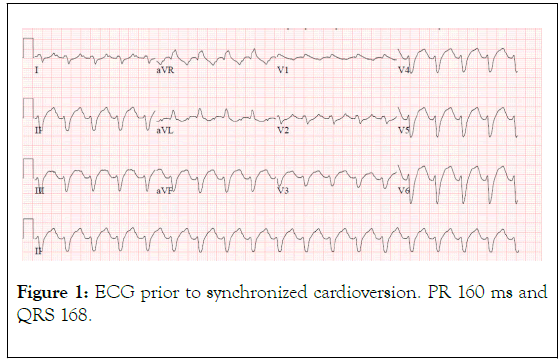
Figure 1. ECG prior to synchronized cardioversion. PR 160 ms and QRS 168.
A repeat EKG was concerning for ischemia in leads II, III, aVF and cardiology was emergently consulted (Figures 2 and 3). They noted the EKG changes were consistent with flecainide toxicity, specifically a prolonged PR interval and QRS (Figures 1 and 2). Emergency bedside echocardiogram revealed that left ventricle was hyperdynamic, small, and under-filled.
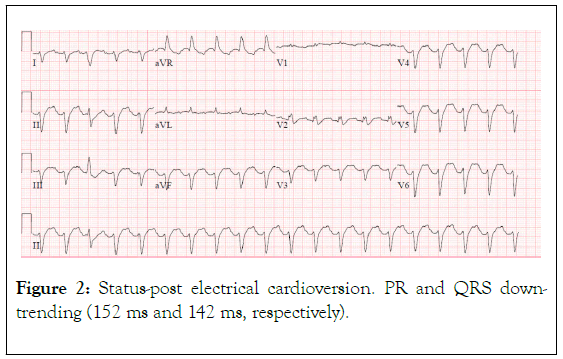
Figure 2. Status-post electrical cardioversion. PR and QRS downtrending (152 ms and 142 ms, respectively).
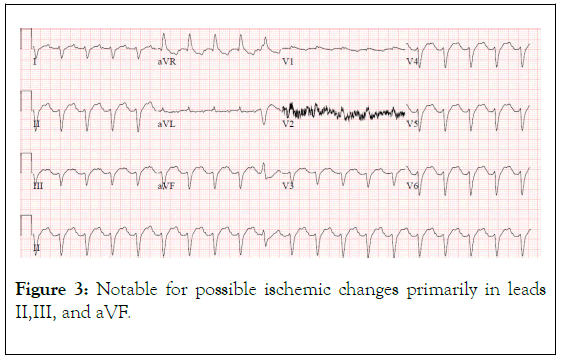
Figure 3. Notable for possible ischemic changes primarily in leads II,III, and aVF.
She became hypotensive and required norepinephrine for blood pressure support and was started on empiric aztreonam and vancomycin for presumed sepsis. She was subsequently admitted to the Medical Intensive Care Unit (MICU). After admission to the MICU, she was started on two more vasopressors and stress dose steroids for septic shock secondary to urosepsis. Of note, she remained unresponsive since intubation despite a head CT that was negative for hemorrhage and receiving no sedatives. She was eventually transitioned from full CPR to do not resuscitate (DNR) as further aggressive therapies were judged to be futile. Four days after presentation, she became bradycardic then developed asystole and died moments later.
Her wide complex tachycardia on admission (Figures 1-3) was ultimately attributed to flecainide toxicity in setting of AKI. The acute kidney injury resolved once the flecainide was discontinued (Figure 4). Two days following her death it was found that her flecainide level was in the toxic range at 2.02 ug/mL (normal range: 0.20-1.00 ug/mL, toxic>1.50 ug/mL).
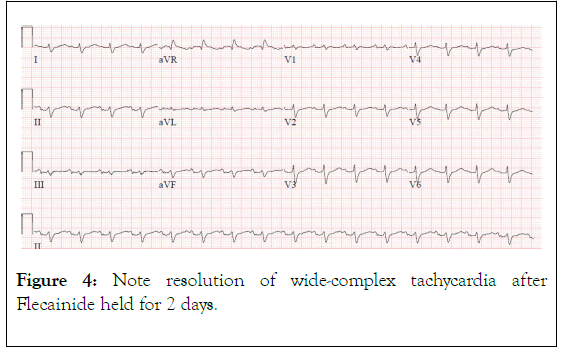
Figure 4. Note resolution of wide-complex tachycardia after Flecainide held for 2 days.
One of the most concerning adverse reactions to flecainide is the proarrhythmia effect associated with its use. This cardiotoxicity risk is believed to be increased by hypokalemia, as was seen in our patient [6,7]. Because of the role of both the liver and the kidneys in the elimination of flecainide, the dosing of flecainide needs to be adjusted in individuals who develop either liver failure or renal failure. In this case the acute kidney injury was likely another factor in developing flecainide toxicity.
Signs of flecainide toxicity include marked prolongation of the PR interval and widening of the QRS duration on ECG, both of which were seen in our patient (Figures 1-3). In cases of overdose, the mortality with class 1c agents has been reported to approach 22% [8] Conduction disturbances can rapidly progress to life‐threatening arrhythmias, electrical depolarization of the heart without myocardial fiber shortening, and asystole. Therefore, early recognition and proper treatment are vital.
Treatment of flecainide cardiac toxicity involves increasing the excretion of flecainide, blocking its effects in the heart, and cardiovascular support to avoid lethal arrhythmias. Recommendations to counteract those adverse effects include the administration of a beta-sympathomimetic agent and administration of a sodium load, often in the form of hypertonic sodium bicarbonate [9]. Extracorporeal Membrane Oxygentation (ECMO) may be required if the arrhythmia leads to significantly decreased cardiac output, and a left ventricular assist device (LVAD) may be needed to increase blood flow to the liver and kidney [10].
Flecainide intoxication is rare but serious due to the potential for cardiogenic shock. Its diagnosis can be difficult as the flecainide serum level may take days to result and there are no pathognomonic clinical signs. This case demonstrates the necessity of keeping flecainide toxicity on the physician’s differential for patients who are taking the drug, as well as what ECG and laboratory findings can suggest it as the etiology. A heightened suspicion is warranted in the patient with new renal impairment. Treatment can be lifesaving if initiated promptly.
Citation: Joshua Newson, Bradford L Walters, Brett R Todd (2020). An Atypical Idiopathic Retinal Vasculitis, Aneurysms and Neuroretinitis IRVAN: Case Report. J Clin Toxicol. 10:433. DOI: 10.35248/2155-9570.20.10.433
Received: 09-Jan-2020 Accepted: 23-Jan-2020 Published: 30-Jan-2020 , DOI: 10.35248/2161-0495.20.10.433
Copyright: © 2020 Newson J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.