Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Review Article - (2015) Volume 5, Issue 6
The data, concerning to the nitrogen oxidation reaction, initiated by electrical discharge, are reviewed in this article. The reviewed data lead to conclusion that critical concentrations of NO molecules and NO3 radicals can be achieved in course of discharge in air at some special conditions, thereafter avalanche-like rise of reaction velocity is observed. At explosion conditions the oxidation of nitrogen proceeds until full exhaustion of atmospheric oxygen in the reaction zone. Explosion like kinetics of nitrogen oxidation results in high concentration of electronically excited molecules NO(B 2Π), it forms conditions for laser generation in the blue region of the spectrum. The generation of this kind was observed. The proposed mechanism of chain reaction explains all the experimental data.
<Keywords: Nitrogen oxidation; NO; NO3 radicals; Chain reaction
“Biological nitrogen fixation contributes about half of total nitrogen input to global agriculture, the rest coming from nitrogenous fertilizer produced chemically from the Haber-Bosh synthesis of ammonia. To produce the hydrogen gas together with the high temperatures and pressures needed for this chemical process, about 1% of the world’s total annual energy supply has to be consumed” [1]. Another possible way to produce fixed nitrogen is the atmospheric nitrogen oxidation in electric discharge. But this way is still not used in industry for the reasons of low energetic efficiency [2]. Discovery of explosion in atmospheric air initiated by an electric discharge [3] generates new hopes to overcome mentioned above problem, if chemical chains are produced at explosion conditions. The review of data, important for performing of nitrogen oxidation chain reaction, is the aim of this article.
Elementary steps of nitrogen oxidation chain reaction
From two stages of nitrogen oxidation chain reaction N + O2 => NO + O and O + N2 => NO + N the second one needs more energy and, for this reason, needs excited N2 molecules:
O + N2(A 3Σ+ u) => NO + N(2D), [4] (1a)
O + N2(v ≥ 13) => NO + N, [2] (1b)
Formation of electronically excited N atoms in the electrical discharge at room temperature was observed in ref. [5]:
N(4S) + N2(A 3Σ+u) => N(2P) + N2 (2)
The excited N atoms are able to react rapidly with O2 molecules [6], forming NO(v ≤ 12) [7] and O atoms. The O atoms are not able to prolong chemical chains without N2(A 3Σ+u) molecules. Thus the reactions
N(2D, 2P) + O2 => NO + O (3)
N2(A 3Σ+u) + O2 => N2 + O + O (4)
should be considered as chain termination reactions. The rapid reaction [8]
N(4S, 2D, 2P) + NO => N2 + O (5)
limits the NO yield in the electrical discharge in N2/O2 mixtures to several percent’s.
The possible role of NO3 radicals
Formation of NO3 radicals in the reaction [9-11]
NO + O2 + M => ON-O-O(•) + M (6)
can enhance the contribution of chain mechanism due to reactions
NO3 + N(2D) => NO(B 2Π, v ≥ 2) + NO2* (7)
NO(B 2Π, v ≥ 2) + N2 => NO + N2(A 3Σ+ u) (8)
Reproduction of N2(A 3Σ+u) molecules can result in new chain initiation reaction (1) and contributes to formation of positive feedback between reactions (1) and (6-8). The positive feedback results in avalanche-like growth of reaction velocity and NO3 concentration according to equation
d([NO3]/dt =?[NO3], (9)
where ? - is a constant, [NO3]- is the concentration of NO3 radicals. The proposition about NO3 radical formation resulted in possibility of explosion-like kinetics of nitrogen oxidation reaction. In this case the avalanche-like growth of NO(B 2Π) radiation intensity and prolongation of oxidation process until full O2 exhaustion are expected.
The proposed mechanism makes use of reactions (7) and (8), which were discussed never before. It can be noted, that the reaction (7) is similar to reaction between NO3 and NO radicals, proposed in ref. [9]. The reaction (7) is in accordance with energy conservation law and with spin conservation rule.
Emission spectra of discharge and afterglow in nitrogen-oxygen mixtures
Several reviews are written on the discharge and afterglows in nitrogen and nitrogen-oxygen mixtures spectroscopy, for example [12]. The most intense bands in the spectra of discharge in nitrogen and nitrogen-oxygen mixtures belong to the First Positive and Second Positive systems of N2. The violet and UV radiation in Second Positive system is excited by electron collisions. Radiative transitions in Second Positive system result in population of N2 excitation level, radiating in First Positive system. 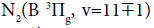 molecules are formed in N atoms recombination reaction.
molecules are formed in N atoms recombination reaction.
Bands of NO β-system can be easily excited in nitrogen with traces of O2. They are produced by N + O recombination reaction [13]. Addition of several percents of oxygen crucially diminishes the β-Bands intensity and rises NO2 emission, produced by NO + O recombination. In discharge in air NO β Bands are not observed, but NO2 emission is very strong [14] (See also Figure 1).
In most kinds of discharges in air NO3 radicals are not present
NO β-system is absent in shirt pulse discharge spectra in air (Figure 1). This indicates to the absence of NO3 radicals, according to reaction (7). This fact is worth attention: reagents for NO3 formation - NO and O2 - are undoubtedly present in plasma. The possible cause for NO3 absence may be too high temperature in discharge, NO3 swiftly decomposes under such conditions. To accumulate NO3 in essential concentration some special sorts of discharge are needed. The discharge should produce as small heating, as possible. Two kinds of discharge, fitting this demand, will be discussed in next section.
Pulse-periodic discharge and discharge, initiated by ferrite surface
We have found two kinds of discharge in air, when the process exhibited features, typical for explosions, caused by chain mechanism of reaction: we observed an induction period several milliseconds long before the explosion itself. Emission spectra belonging to the induction period and to the explosion, were different. The estimation of heating at the pre-explosion stage gave the value: several centigrade. Such small value does not permit to explain the transition to the explosion stage as a result of temperature enhancement [3]. The emission spectrum at the explosion stage belonged to NO(B 2Π) molecules, and that is not typical for discharge in air spectra (see above). Both sorts of discharge under consideration fit the demand about temperature minimization. It is more obviously in the case of pulse periodic discharge, excited by a high-voltage supply with average power about 5 W and frequency 1000 Hz (the electrical circuit, used for pulse-periodic discharge excitation, is placed on the Figure 2).
A sequence of negative pulses came from pulse-generator to the grid of a valve. The negative voltage on the grid locked the valve: its anode current declined from the value about 0.5 A to 0. It resulted in generation of high voltage (about 5 kV) pulse on the induction coil L (Figure 2). It caused spark discharge ignition in both electrode gaps, shown on Figure 2: in the main gap, marked with “A” letter, and in subsidiary one, marked with “B” letter. One frame of video recording is placed on Figure 3, this frame contains the image of the pulseperiodic spark discharge, obtained with apparatus, described on Figure 2, with one difference: the grounded electrode of the main gap “A” is not sharpened, but is made of a smooth aluminum piece. Shirt circuit between the electrodes of gap “B” (or swift nearing them) results in flash between the electrodes of gap “A” (Figure 4). The diameter of the observed luminescent cloud is about several centimeters (Figure 4).
Loss of heat during intervals between the discharge pulses makes for keeping down the gas temperature in the discharge zone. Initiation of discharge by ferrite surface results in achievement of record-breaking small value of E/n [15]: with voltage 440 V, applied to ferrite surface between electrodes, removed to 5 mm from each other, it makes E 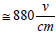 . When relation of electric field tension E (in V/cm) to the gas density n (in Torr) equals 1 (that is close to our case), only about 0.1% of total discharge energy transforms to “swift heating” (that is “before any contribution of V-T and E-T processes”) [16].
. When relation of electric field tension E (in V/cm) to the gas density n (in Torr) equals 1 (that is close to our case), only about 0.1% of total discharge energy transforms to “swift heating” (that is “before any contribution of V-T and E-T processes”) [16].
One frame of video, containing the image of luminescent cloud of explosion in air, initiated by ferrite surface, is placed on Figure 5.
Figure 5: Frame of video, containing the image of luminescent cloud of explosion in air, initiated by ferrite surface. The outline of arrangement of ferrite piece (1) and two electrodes (2 and 3), connected to capacitors (charged to the voltage 440 V), is made with white lines over the image. Explosion sprang up, just when both electrodes touched the ferrite. A white arrow is pointing to the dark field near the middle of the cloud. It means, that, when the gas traveled from the plasma zone between the electrodes to periphery of the cloud, the luminescence intensity enhanced markedly in absence of electric field (that is: due to chemical chain reaction). Presented below experimental data point to chain mechanism of chemical reaction, connected with explosion in air.
Oscillograms of radiation from explosion in air and of current in it
Oscillograms of radiation and current were obtained for the explosion in air, initiated by single pulse discharge on the ferrite surface. For details of recording oscillograms see ref. [3]. Figure 6 displays one frame of the video record, which shows 6 oscillograms and the zero line, the result of the imposition of a set of oscillograms recorded before the appearance of the signal. In Figure 6, the zero line is positioned above the other oscillograms. Bringing the ends of electrodes into contact with the surface of the ferrite starts the discharge (emission occurs, as evidenced by the downward deviation the oscilloscope trace from the zero line). This stage of the process corresponds to oscillograms 1-5, with the radiation intensity increasing with time (oscillograms are increasingly shifted downward from the zero line). Finally, at approximately the middle of oscillogram 6, the trace begins to move vertically downwards, which corresponds to the development of an explosion. The subsequent frames of the video recording show a smooth return of the oscilloscope trace to the zero line within a few milliseconds after the explosion.
Figure 6: Oscillograms of the explosion in air chemiluminescence signal. The zero line is the result of superposition of many traces in the absence of the photosignal, it is the bright line located at the top. With increasing photosignal, the oscilloscope trace shifts downwards (traces 1-5). Trace 6 shows the moment when the oscilloscope trace shifts downward almost vertically. This point corresponds to the onset of the explosion. The continuous trigger mode with a sweep rate of 50 μs/cm is used: i.e., a single pass of the trace on the screen takes about 500 μs, whereas the record of six oscillograms lasts for 3-6 ms.
To examine the evolution of the discharge current, the negative terminal of the capacitor bank was connected with the respective electrode of the discharge gap through the primary winding of a transformer, the secondary winding of which was connected to oscilloscope input. For more details see ref. [3]. The oscilloscope operated in the continuous trigger mode at a sweep speed of 5 ms/cm, with video recording of the oscilloscope screen. A typical oscillogram is displayed in Figure 7. This oscillogram shows an induction period (the beginning and end of which are marked by white arrows): the discharge current during this period is rather small, it provides a deviation of the oscilloscope trace from the zero line of about half the width of the trace. The induction period is followed by a sharp increase in the current to a maximum value, after which it decays to zero. The duration of the induction period is about 5 ms, while the decay of the current from maximum to half-maximum takes 2 ms. Thus, the temporal characteristics of the induction period and the explosion period derived from the time histories of the radiation intensity and electric current are in good agreement.
Based on the known charge on the capacitor (C=1000 μF, U=220 V, and q=CU=0.44 C on two capacitors) and the discharge time, it is possible to estimate the average discharge current: J=220 A. For the peak current can be reasonably estimated as 400 A. Assuming that the current during the induction period is 100 times less than its peak value (In agreement with the observed oscilloscope signals, 0.5 and 50 mm, respectively), we can evaluate the current during the induction period for single pulses as J=4 A. For the indicated capacitance of the capacitors and the voltage on them, the amount of energy input into discharge is ~ 50J.
The oscillograms of the explosion in air radiation shown in Figure 6 make it possible to estimate the highest temperature reached in the discharge zone by the end of the induction period. To do this, we made use of the fact that, during the induction period, the emission intensity signal increases by about 1.5-fold for after each pass of the trace across the oscilloscope screen. That is, over the entire induction period, the intensity increases by about an order of magnitude. Considering the radiation equilibrium and the NO concentration constant, we can estimate the preexplosion heating ΔT required for this increase as follows: ΔT=2.3RT2/E, where E=6 eV=144000 cal/mol, T=300 K, R=2 cal/(mole K). As a result, we obtain ΔT~3K. The resulting estimate of the heating of the plasma by the end of induction period enabled to reject any mechanism involving an increase in temperature as the cause of the explosion; rather, it could be the presence of a positive feedback between the concentration of electronically excited molecules and the ionization rate, with the most important contribution coming from the process of associative ionization involving N(2P) metastable nitrogen atoms [17]: N(2P) + O => NO+ + e-.
Oscillograms of the radiation intensity and of the discharge current confirm the presence of preexplosion induction period, during which the chemical process already takes place, but its intensity is about two or three orders of magnitude less, than during the explosion. Such kinetics is typical for branched chain chemical reactions.
Emission spectrum of the explosion in air, initiated by the discharge near the ferrite surface
For the experimental details of recording the spectrum see reference [3]. The emission spectrum of the explosion in air, initiated by the discharge over the ferrite surface is placed in Figure 8. All the observed bands are present in the emission spectrum given in ref. [13] and identified as β-bands of the NO molecule.
Emission of N2 and NO2 molecules is not present in the spectrum on the Figure 8. It indicates that N and O atoms concentrations are too small. Recombination reaction N + O can not explain NO β- bands under this conditions. Reaction (7) is a possible source of NO(B 2Π) molecules. Reactions (7),(8) and (1) make up a chain reaction. Electronically excited molecules NO(B 2Π) and N2(A 3Σ) are formed in each link of that chain. Such situation is promising for chemical lasers. It should be noted, that NO β-Bands in the blue and green regions of spectrum correspond to optical transitions into high vibrationally excited levels. Such levels are characterized by high rate of V-V and V-T exchange. This makes for population inversion at mentioned transitions. The laser generation of NO(B 2Π) molecules in blue region is observed under conditions of explosion in air [15]. This fact confirms to the assumption about chain mechanism of NO formation reaction.
Manifestations of chain reaction, initiated by pulse-periodic discharge
In this section the experimental data are presented, obtained after publication of articles [3,15]. These experiments were designed with aim to obtain more evident demonstrations for chain mechanism of nitrogen oxidation. The data placed on Figures 5 and 9-11 are published in ref. [18].
First of all it was needed to demonstrate the prolongation of chemical luminescent reaction during the intervals between pulses of high voltage upon the conditions of pulse-periodic spark discharge. The camera used needed about 30 ms to make a frame, but each interval between discharge pulses lasted only about 0.5 ms. To get some knowledge about what is going on during discharge intervals, we recorded video by moving camera. To do this we placed the camera on a swing: the swing board was hanged up on a hook, positioned about 4 m above the board level. During recording the camera moved like a pendulum. The amplitude of camera swinging was about 5 m. To produce the explosion just at a moment, when camera moved through the lowest point of trajectory, the shirt circuit was made between the electrodes of the gap “B” (see Figure 2) at this moment. It resulted in explosion in the gap “A” (Figure 2). The initial dimensions of the gaps “A” and “B” were so, that pulse-periodic sparking was observed in both gaps.
The frame of video on Figure 9 was made by method described above. Two traces (1 and 2) can be seen on the Figure 9. The trace 2 contains only the spark image. Trace 1 contains the image of explosion in the middle of frame. The progress in time corresponds with movement from right to left. The right white arrow points to the last dark interval before the explosion. From that moment until the exhaustion of chemical reaction no dark intervals are to observe. The induction period several milliseconds long can be noted again (its finish is marked by left white arrow in Figure 9). It can be concluded, that during the explosion the luminescence intensity is not sensitive to the electric field in the gap (it is two or three orders of magnitude more than in spark discharge). The explanation of luminescence during the intervals between high voltage pulses is based on assumption about chemical chain reaction mechanism (reactions (1, 7-8)).
Estimation of NO2 concentration achieved in explosion in air
To obtain an estimation of NO2 concentration produced in explosion in air, initiated by single discharge near the ferrite surface, experimental arrangement, shown in Figure 10, was used.
Figure 10: Outline of the experimental arrangement, used for estimation of NO2 concentration. Designations: 1: The light source with LED’s; 2: T-pipe of ½ inch in diameter, 3: Sharp boundary of the light spot on the white screen; 4: White screen; 5: Electrode, touching to the ferrite peace; 6: The peace of ferrite.
Electric discharge near ferrite surface inside the T-pipe (see Figure 10) resulted in color change of the light spot on the white screen (4): before the discharge that spot was white-blue; after the discharge it’s color changed to green-yellow (see Figure 11). It can be explained by full absorbing of violet and blue components of light by NO2. Investigation of the frame in Figure 11 in blue light shows, that the circle boundary of the light spot on the white screen become indistinguishable, that fact indicates on full disappearing of blue light. Based on data about absorbing coefficient of NO2 [19], NO2 concentration was found to be 5 × 1018 molecules/cm3. This value is approximately equal to O2 concentration in air in agreement with assumption about full exhaustion of oxygen in the explosion zone due to chain mechanism of nitrogen oxidation.
On the role of electronically excited molecules in the mechanism of NO oxidation
NO oxidation takes place at later stage of nitrogen oxidation. For this reason an independent investigation of NO oxidation was expected to give new information about N2 + O2 reaction. It is known [20] that NO oxidation – is a spontaneous process, its characteristic time is about 0.1 s. Such velocity is not enough to observe the luminescence of electronically excited molecules. It was found, that it is possible to enhance the reaction rate by its stimulation by visible radiation of red color. Below is to find the description of experiments on photochemical stimulation of NO oxidation. These experiments were published in ref. [21,22]. The outline of experimental arrangement used for investigation of He-Ne laser (wave length 0.63 μm) irradiation effect on the active gas mixture, obtained by action of nitric acid on copper is placed on Figure 12.
Figure 12: An experimental arrangement used for investigation of He-Ne laser irradiation effect on the active gas mixture, obtained by action of nitric acid on copper. Designations: 1: He-Ne laser; 2: Syringe; 3: Nitric acid; 4: Reaction tube; 5: Tube made of copper, it is placed exactly below the syringe; 6: Place of the fan. The binary arrow shows the direction of air stream from the fan.
By using the syringe (2) several drops (about 0.3 ml) of concentrated nitric acid were introduced into copper tube (5). After that clouds of brown gas filled the reaction tube (4). Turning on the fan to the right from the reaction tube resulted in appearance of visible red trace of He-Ne laser beam inside and outside the reaction tube (see Figure 13). Without fan the laser beam was not visible. Three possibilities for explanation of observed laser beam trace were considered: 1) scattering of laser radiation by smog particles (formed of N2O5); 2) fluorescence of NO2; 3) chemical luminescence. Only the last possibility enables to explain all observed facts. The most intriguing of them is observing a new laser beam branching off from the trace of He-Ne laser beam (see Figure 14). This branching off laser beam is produced by stimulated emission of radiation, coming from NO2 electronically excited molecules.
Such molecules are produced by chain reaction:
O + NO + M => NO2** + M (10)
NO2** + NO3 => NO2* + NO2 + O (11)
NO2* + NO3 => 2NO2 + O (12)
The chain reaction (10-12) produces O atoms and NO2 electronically excited molecules in exponentially rising numbers, it explains, why instead of expected extinction of laser beam in the reaction tube the branching off a new beam is observed.
The low power pulse-periodic spark discharge and the discharge initiated by ferrite surface permit to observe the chain reaction of nitrogen oxidation in the form of an explosion in air. NO oxidation (including reactions (10-12)) gives a source of O atoms for chains prolongation reaction (1). Reactions (6-8) reproduce N2(A 3Σ) molecules. Reactions (1, 6-8) together with (10-12) give description of chain reaction responsible for explosion in air. Reactions making up a possible mechanism of nitrogen oxidation chain reaction are collected in Table 1.
| 1 | O + N2(A3Σ) => NO + N(2D) | [4] |
| 2 | NO + O2 + M => NO3 + M | [9] |
| 3 | NO3 + N(2D) => NO2 + NO(B2Π) | Newly proposed |
| 4 | NO(B2Π) + N2(v=1,2) => NO + N2(A3Σ+u) | Newly proposed |
| 5 | N(2P) + O => NO+ + e- | [17] |
| 6 | NO + O + M => NO2** + M | [22] |
| 7 | NO3 + NO2** => NO2 + NO2*+ O | Newly proposed |
| 8 | NO3 + NO2* => 2NO2 + O | Newly proposed |
Table 1: Mechanism of nitrogen oxidation chain reaction.
Using of ferrite core for fly-back transformer, which is about 70 mm in length, makes it possible to produce explosion in air in such a volume, which is enough for laser generation in blue region of spectrum [15]. This fact makes evident efficiency of chain reaction in producing electronically excited NO molecules. The measurement of chain reaction efficiency in producing nitrogen oxides remains a task for future work.