Journal of Medical Diagnostic Methods
Open Access
ISSN: 2168-9784
ISSN: 2168-9784
Review Article - (2020)Volume 9, Issue 1
Basaloid follicular hamartoma is cogitated as an exceptional, benign, cutaneous, hair follicular hamartoma essentially engendering solitary, linear or diffusely disseminated papules and plaques. Brown et al initially scripted and characterized basaloid follicular hamartoma in 1969 as a generalized follicle hamartoma with significant basaloid cell proliferation ambiguously confined to the entire hair follicle. Antecedent lesions were composed of multiple, flesh coloured, hyper-pigmented papules with dissemination upon the face, neck, trunk, extremities, scalp, nasolabial fold, peri-orbital area, palmar and solar pits along with concurrence of diffuse alopecia, milia, comedones, myasthenia gravis or various inherited syndromes. Basaloid follicular hamartoma as a terminology was initially adopted by Mehregan in 1985 which essentially describes a novel, cutaneous adnexal neoplasm which can arise spontaneously or is associated with an underlying disorder or a distinct, inherited syndrome.
Cutaneous; Papules; Basaloid; Follicle; Neoplasm
Disease characteristics
• Basaloid follicular hamartoma is contemporarily categorized into clinical variants enunciating
• Solitary or multiple papules
• Localized papules or plaques with accompanying alopecia
• Localized linear papules or plaques
• An autosomal dominant, generalized, familial variety devoid of associated systemic conditions
• Generalized papules with adjunctive alopecia and myasthenia gravis
Clinical representation of the exceptional, benign, adnexal, hair follicular neoplasm is diverse and it appears as a familial syndrome, a congenital condition or an acquired disease [1,2]. Histological distinction betwixt basaloid follicular hamartoma and basal cell carcinoma is challenging. The disorder carries a nomenclature of basaloid follicular hamartoma or a generalized basaloid follicular hamartoma syndrome and emerges as an autosomal dominant disorder. A juvenile variant is also exemplified as linear unilateral basal cell nevus associated with comedones, which demonstrates a linear distribution, usually along lines of Blaschko. Generalized hair follicle hamartoma or multiple basaloid follicular hamartoma with concurrent alopecia and myasthenia gravis are additional terminologies [1,2]. Although basaloid follicular hamartoma is not categorized as a premalignant lesion, intense monitoring is necessitated to exclude progression to overt basal cell carcinoma. Subjects with basal cell nevus syndrome demonstrate an enhanced possibility of malignant conversion. Lesions occurring in concordance with autoimmune disease can be therapeutically managed to resolve concurrent autoimmune disorders [2,3].
Clinical Elucidation Basaloid follicular hamartoma demonstrates diverse clinical representations and can appear as localized or generalized. Characteristically, localized or disseminated, multiple, tan or dark brown, homogenous, warty, papules varying from one millimetre to three-millimeter dimension usually emerge upon on the cutaneous surface of the face, scalp, neck, axilla, trunk, limbs and pubic region and demonstrate an inferior cosmetic outcome. Solitary or multiple, hypo-pigmented to hyperpigmented papules can appear upon the centroidal face. Additionally, plaques or coalescing papules can be engendered. Basaloid follicular hamartoma can be associated with alopecia, hypertrichosis or open comedones. Unilateral lesions can follow lines of Blaschko and delineate associated neurological features [3,4]. Concurrent medical conditions such as atrial fibrillation, chronic kidney disease, dilated cardiomyopathy obstructive sleep apnoea, congestive heart failure or hyperlipidaemia are observed. Papules are significantly enunciated upon nasolabial folds, forehead, pre-auricular and periorbital region. Plaques engendered with basaloid hamartoma syndrome can simulate lesions of nevus sebaceous, lupus erythematosus or sarcoidosis. Basaloid follicular hamartoma is associated with specific autoimmune diseases such as myasthenia gravis, alopecia, systemic lupus erythematosus, palmar pits, hypohidrosis, hypotrichosis, cystic fibrosis, Happle-Tinschert syndrome, X-linked dominant Bazex syndrome or Bazex- Dupre- Christol syndrome, nevoid basal cell carcinoma syndrome or Gorlin syndrome or basal cell nevus syndrome. Additionally, generalized basaloid follicular hamartoma syndrome with an autosomal dominant pattern of disease penetration and Brown- Crounse syndrome is associated with basaloid follicular hamartoma [3,4]. Basaloid follicular hamartoma associated with an autoimmune disorder can depict a regression of cutaneous lesions in instances appropriately treated for accompanying autoimmune conditions [3,4].
Basaloid follicular hamartoma arises sporadically or on account of mutation of patch (PTCH) gene situated upon chromosome 9q23, the gene which engenders nevoid basal cell carcinoma syndrome. Essentially benign basaloid follicular hamartoma demonstrates a milder genomic mutation of PTCH gene with the encoding of a receptor that adheres to the product of sonic hedgehog (SHH) gene. Several signaling proteins associated with chromosomal patterning during embryonic development are encoded with sonic hedgehog (SHH). PTCH receptor generally adheres to G- protein smoothened (SMO) [4,5]. In subjects devoid of the sonic hedgehog gene, PTCH receptor can immobilize the smoothened (SMO), thereby inhibiting a variety of downstream signaling. Conditions of sonic hedgehog adherent to PTCH receptor, smoothened (SMO) is uninhibited and downstream hedgehog target genes are upregulated through a cascade employing Gli family transcription factor. Constant molecular signaling ensures an unsupervised and aberrant cellular growth and division [2,3]. Mutations of protein patched homolog (PTCH) gene incur disrupted features of tumour suppression. Aberrant PTCH gene adheres to a sonic hedgehog which generates a protein signal incriminating embryogenesis Constitutive activation of downstream signaling proteins are involved with Gli transcription factors with the consequent occurrence of anomalous tissue growth [2,3]. Targeted next-generation sequencing is beneficial in determining syndromic phenotypes of basaloid follicular hamartoma. PTCH1 gene situated upon chromosome 9q23 is implicated in generalized basaloid follicular hamartoma syndrome (GBFH) and Gorlin-Goltz syndrome. Basaloid follicular hamartoma occurring within aforesaid syndromes indicates that the specific disorders constitute a disease continuum. Basal cell nevus syndrome can arise on account of PTCH2 mutation or a SUFU genetic mutation which is essentially an identical signalling molecule. However, in contrast to generalized basaloid follicular hamartoma basal cell nevus syndrome is denominated by jaw keratocysts, calcification of falx cerebri, bifid ribs and macrocephaly [4,5]. Aforesaid genetic mutation is additionally cogitated in the genesis of Nevoid Basal Cell Carcinoma Syndrome (NBCCS), although the manifestations are minimal [4,5].
Lesions demonstrating an increase in magnitude or altered external appearance necessitate a cogent tissue evaluation. Basaloid follicular hamartoma is characterized by anastomosing strands and cords of proliferating epithelial and basaloid cells. Miniature collections of basaloid cells articulate a continuum of strands or cords and cystic structures. The lesion is devoid of stroma- epithelial retraction. Anastomosing strands and cords of basaloid cells in the absence of retraction betwixt the stroma and tumour epithelium and a CD34+ immune reactive stroma is indicative of basaloid follicular hamartoma [5,6]. Basaloid follicular hamartoma generally arises within regions demonstrating normal hair follicles. Anastomosing strands and cords of basaloid and squamous epithelial cells demonstrate connectivity to superimposed squamous epithelium. Cellular content of the benign, adnexal neoplasm is comprised of bland cells devoid of atypia, mitosis and apoptotic bodies. The intervening stroma is loosely arranged, cellular or myxoid. Additionally, horn cysts and foci of pigmentation can be exemplified. Peripheral palisading and stromal-epithelial retraction can be represented although it is usually focal and minimal, in contrast to the alterations typically enunciated within basal cell carcinoma [6,7]. Basaloid cells comprise of hyperchromatic nuclei, infrequent mitotic figures, scanty cytoplasm and a minimal, intervening stroma is delineated betwixt the cellular aggregates (Figures 1-8). Anastomosing strands and cords of basaloid cells are generally exhibited within the incriminated hair follicles. As basaloid follicular hamartoma is primarily restricted to the hair follicles, intervening tissue within the dermis, inter-follicular region and reticular dermis can be histologically normal. Nevertheless, neoplastic cells can incriminate and decimate pre-existing hair follicles, inter-follicular dermis or occasionally infiltrate deep-seated dermal regions [2,3,8].
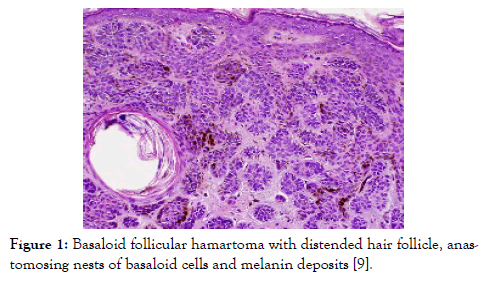
Figure 1: Basaloid follicular hamartoma with distended hair follicle, anastomosing nests of basaloid cells and melanin deposits [9].
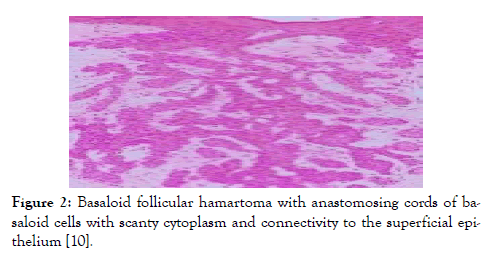
Figure 2: Basaloid follicular hamartoma with anastomosing cords of basaloid cells with scanty cytoplasm and connectivity to the superficial epithelium [10].
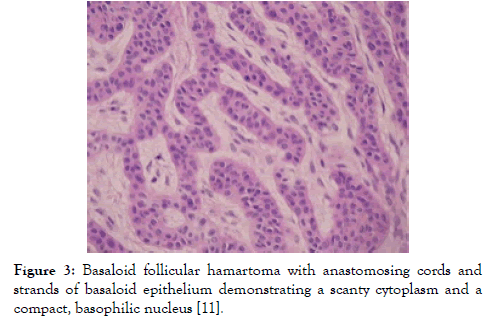
Figure 3: Basaloid follicular hamartoma with anastomosing cords and strands of basaloid epithelium demonstrating a scanty cytoplasm and a compact, basophilic nucleus [11].
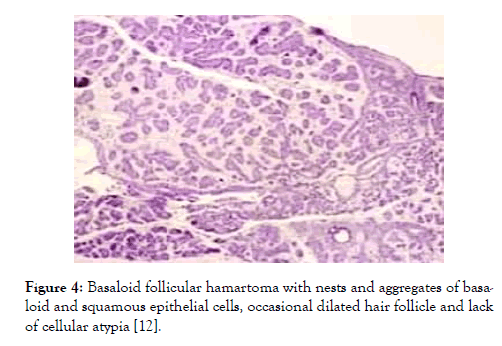
Figure 4: Basaloid follicular hamartoma with nests and aggregates of basaloid and squamous epithelial cells, occasional dilated hair follicle and lack of cellular atypia [12].
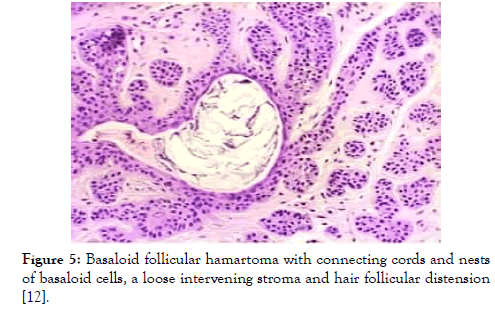
Figure 5: Basaloid follicular hamartoma with connecting cords and nests of basaloid cells, a loose intervening stroma and hair follicular distension [12].
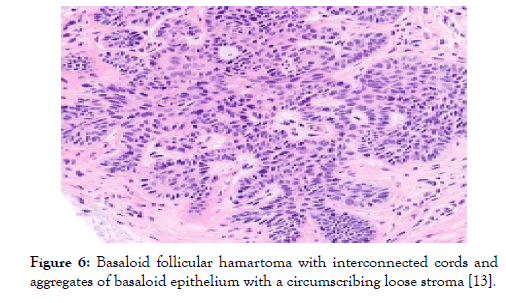
Figure 6: Basaloid follicular hamartoma with interconnected cords and aggregates of basaloid epithelium with a circumscribing loose stroma [13].
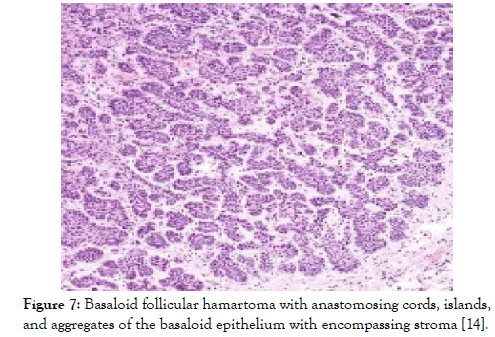
Figure 7: Basaloid follicular hamartoma with anastomosing cords, islands, and aggregates of the basaloid epithelium with encompassing stroma [14].
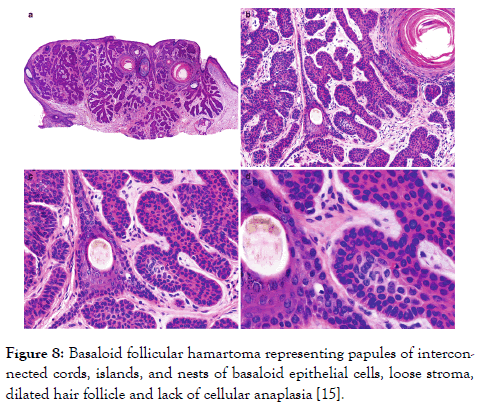
Figure 8: Basaloid follicular hamartoma representing papules of interconnected cords, islands, and nests of basaloid epithelial cells, loose stroma, dilated hair follicle and lack of cellular anaplasia [15].
Basaloid cells are immune reactive to B-cell lymphoma 2 (Bcl-2) and non-reactive to androgen receptor antigens. The intervening stroma is immune reactive to CD34+, whereas CD20 displays a scattered immune reactivity within the tumour. Immune reactivity exemplified with Bcl-2 is pertinent to reaction with peripherally dispersed basaloid cells, although diffuse staining is delineated within basaloid cells of basal cell carcinoma. Immune reactivity to CD34+ demonstrates stromal staining enveloping the basaloid cells. Besides, the stroma of basal cell carcinoma infrequently reacts with CD34+. CD10+ is immune reactive to stroma circumscribing basaloid cells of basaloid follicular hamartoma whereas stroma and basaloid cells constituting basal cell carcinoma react to CD10+ [2,3]. p40 is intensely reactive in basaloid cells whereas a focal reactivity to CK20 is exemplified in basaloid follicular hamartoma. Nevertheless, the exceptionally discerned basaloid follicular hamartoma is devoid of a specific immune marker. Thus, cogent immunehistochemical reactions are not a viable option for diagnosing a basaloid follicular hamartoma. Basaloid follicular hamartoma can be observed on frozen section especially during the adoption of Moh’s micrographic surgery [2,3]. Morphological parameters for discerning basaloid follicular hamartoma on the frozen section are identical to those observed on haematoxylin and eosin-stained paraffin- embedded sections. Nevertheless, distinction betwixt basaloid follicular hamartoma and basal cell carcinoma employing a frozen section is challenging.
Considerable morphologic concurrence of basaloid follicular hamartoma is enunciated betwixt benign tumours of hair follicular infundibulum and infundibulocystic basal cell carcinoma. The distinction of basaloid follicular hamartoma is partly contingent to the articulation of solitary or multiple papules or plaques. Segregation is mandated from basal cell carcinoma, sebaceous hyperplasia, seborrheic keratosis, trichilemmoma, intradermal melanocytic nevus, syringoma, angiofibroma, steatocystoma nevus sebaceous, lichen striatus, linear epidermal nevus, associated benign, basaloid dermal tumours and basal cell nevus [2,4]. Basaloid follicular hamartoma requires a demarcation from trichoepithelioma or trichoblastoma which is a benign tumour composed of distinct islands of basaloid cells with peripheral palisading and papillary mesenchymal bodies. The emergence of distinct, spherical, cellular islands as opposed to multiple, branching and anastomosing cellular cords are indicative. Tumours of follicular infundibulum demonstrate a growth pattern essentially disseminated along the dermal-epidermal junction, identical to a superficial basal cell carcinoma with fenestrated zones. Also, appearance of pale- staining, glycogen impacted cells are suggestive of trichilemmoma [3,4]. Pilar sheath acanthoma delineates cystic dilatation of hair follicles along with the occurrence of acanthotic, radiating epithelial projections. Trichofolliculoma depicts a centric, enlarged, distended, hair follicle enveloped by secondary, miniature hair follicles [1,2]. Basaloid follicular hamartoma requires a distinction from adjunct epithelial neoplasm demonstrating a malignant potential such as infundibulocystic basal cell carcinoma. Infundibulocystic basal cell carcinoma can display a similar morphology to basaloid follicular hamartoma although cellular atypia and mitosis is enhanced. Stromal-epithelial retraction is prominent in basal cell carcinoma which also depicts a myxo-inflammatory exudate. Clinical concordance and cogent immune- reactions can assist the demarcation. Stromal cells of basaloid follicular hamartoma are immune reactive to CD34+, delineate a low proliferative index to Ki-67 and Bcl-2 is inconsistently immune non-reactive whereas basal cell carcinoma is immune non-reactive to CD34-, is intensely reactive to Ki-67 and Bcl-2 is typically immune reactive. Basaloid follicular hamartoma displaying a linear distribution of lesions can recapitulate conditions such as linear epidermal nevus, lichen striatus, linear morphea and basal cell nevus [1,2].
Lesions can be adequately treated with pulsed dye laser (PDL) therapy within the incriminated areas such as the face, temple, cheek or entire forehead. Treatment of basaloid follicular hamartoma lacks a standard of care and therapeutic options are largely limited and experimental. Employment of 5-aminolevulinic acid (5-ALA) is a cogent modality which can be adopted in combination with photodynamic therapy. Aforesaid combination is a safe and preferential therapy in children incriminated with multiple basaloid follicular hamartoma [6,7]. Subjects with basal cell nevus syndrome demonstrating extensive basal cell carcinoma and basaloid follicular hamartoma can be administered 5-aminolevulinic acid and photodynamic therapy for appropriate cosmetic outcomes. Oral and topical retinoid application is efficacious in singular, isolated instances [6,7]. Pulsed dye laser (PDL) is traditionally employed to treat vascular lesions such as port-wine stain, acne, scars and for photorejuvenation. Pulsed dye laser is provided with a wavelength of 595 nanometres which effectively penetrates up to one millimetre to two millimetres into the epidermis, although enlarged spots necessitate an enhanced depth of penetration [8,9]. Apart from traditional treatment for vascular lesions, non-vascular or pigmented lesions or basal cell carcinoma can be efficaciously treated with pulsed dye laser. Pigmented lesions such as solar lentigines, nevus spilus or melanocytic nevus can be appropriately managed with pulsed dye laser which selectively targets and denatures the chromophore of melanin or haemoglobin. The pulsed dye laser is also beneficial in treating verruca vulgaris, molluscum contagiosum, and several non-vascular lesions. Laser decimates circumscribing, miniature vasculature supplying the lesions thereby decreasing nutrients available to incriminated tumour cells. IdenIdentical methodologies of therapeutic degradation of basal cell carcinoma with a pulsed dye laser are exemplified. The pulsed dye laser is advantageous in alleviating acquired, facial basaloid follicular hamartomas. Mechanics of destruction of basaloid follicular hamartoma is on account of selective photo-thermolysis of the vasculature as well as targeting tumour-specific melanin pigment [8,9]. Traditional vascular lasers can be employed to treat basaloid follicular hamartoma such as a potassium- titanyl- phosphate laser and Intense Pulsed Light (IPL) therapy. Additionally, a laser that focuses upon deeper dermal pigmentation can be advantageous, particularly ruby, alexandrite or neodymium-doped yttrium Aluminum garnet lasers. Surgical extermination of the lesions, carbon dioxide laser therapy, and dermabrasion can be beneficially adopted to treat unilateral, facial basaloid follicular hamartoma. However, efficacy and limitations of aforesaid lasers necessitate additional evaluation [8,9].
A competent clinical and histological interpretation is mandated to circumvent unrequired surgical intervention for excision of primarily benign lesions.
Citation: Bajaj A (2020) The Development Rarity- Basaloid Follicular Hamartoma. J Med Diagn Meth 9:289. doi: 10.35248/2168-9784.9.1.289
Received: 20-Feb-2020 Accepted: 05-Mar-2020 Published: 12-Mar-2020 , DOI: 10.35248/2168-9784.9.1.289
Copyright: © 2020 Bajaj A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : None