Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research - (2022)Volume 10, Issue 7
Aim: The aim of this study was therefore to determine if certain lactic acid bacterial species are more efficient at digesting lactose through fermentation, and therefore possibly more prone to have clinical benefit in for the lactose intolerant population. This will be assessed by measuring the change in pH of the bacterial culture as a result of lactic acid production over a period of 405 minutes.
The purpose of the present study was to investigate the effect of a lactose-rich environment on 8 different acidophile bacterial species. The study was designed to try to determine whether certain species are more efficient lactose metabolisers, and therefore more prone to potentially provide clinical benefit when used as a supplement to promote the breakdown of lactose in the small intestine, especially in lactose-intolerant individuals. This was assessed by evaluating and comparing the change in pH of a lactose-based bacterial culture for each species over an 8-hour period. The variation of pH was used as an indirect measure of lactose fermentation and the subsequent production of lactic acid. The experiments performed suggest that Lactobacillus casei is the most effective lactose digesting species, as it triggered the greatest pH decrease, considering a 2.55 difference between the initial and final pH measurements. These findings help to establish the foundation for further clinical and nutritional research evaluating the potential role of this microorganism as an additive in food products and as probiotic supplement directed at lactose-intolerant individuals, either in conjunction with or as substitute to the currently used synthetic lactase.
Lactobacillus casei; Bifidobacterium species; Lactose intolerance; Lactic acid; Probiotic; Fermentation
Lactose and lactase
Lactose is a disaccharide formed within the Golgi apparatus of the epithelial cells of mammary glands as a result of the condensation of glucose and galactose by the enzyme lactose synthase. As the main carbohydrate contained in mammalian milk, it is the most important source of energy during infancy [1].
Lactase, an enzyme located in the microvilli of the small intestine enterocyte is responsible for the digestion of dietary lactose, by hydrolyzing it into glucose and galactose [2]. It’s production is controlled by expression of the Long Chain Triglyceride (LCT) gene [3].
Lactose intolerance
Lactose Intolerance (LI) is a disorder caused by lactase absence or deficiency, leading to impaired lactose digestion [2]. According to the American National Health Research Institute, the main concern associated with it is its potential to reduce or eliminate one’s dairy intake, leading to vitamin D and calcium deficiency. The distribution of LI is geographically uneven, being present in up to 15% of northern European descent, 80% of blacks and Latinos, and 100% of American Indians and Asians [1]. The diagnosis of this condition is made through the hydrogen breath test or through a blood glucose test, performed following the ingestion of lactose [4].
There are three types of LI, with varying degrees of severity. Adult hypolactasia, also referred to as lactase non-persistence, is the most common affecting 75% of the human population. It is caused by the gradual decline in expression of the LCT gene after 2-12 years of age (Deng et al., 2015). With the exception of humans, it occurs naturally in all mammals after weaning, as there ceases to be a purpose for lactose digestion [1]. The secondary type of hypolactasia occurs following gastrointestinal illness due to damage to the small intestine microvilli [3]. In both types described above, one still retains a low lactase activity and therefore is able to tolerate limited ingestion of lactase [1]. Lastly, its rarest and most severe form is congenital lactase deficiency or alactasia, with only 40 reported cases, mainly in Finland. It is caused by an autosomal recessive mutation in the LCT gene, leading to a lifelong absence of lactase [5].
The absence or deficiency of lactase in the small intestine is directly related to the symptoms of this disorder. Unabsorbed lactose osmotically attracts water to the bowel lumen. As it reaches the colon, it is fermented by microbiome bacteria to produce monosaccharides, forming organic acids and excess gases such as carbon dioxide and hydrogen as by-products. This excessive of gas production in turn leads to the development of abdominal pain, flatulence and bloating. Furthermore, the produced monosaccharides cannot be absorbed by the colonic mucosa, leading to increased osmotic pressure, which drives more water into the colon, giving rise to diarrhea. Symptoms typically arise between 30 minutes and 2 hours after the ingestion of lactose [6].
Modern descendants of hunter-gatherer populations, and most human populations before the Neolithic Revolution were lactose intolerant, due to lactase non-persistence. Nevertheless, high environmental pressure associated to a gene-culture co-evolution led to the propagation of an autosomal dominant mutation in the MCM6 gene, which controls the LCT gene, leading to continued production neonatal levels of lactase after weaning [3,7]. Genetic studies suggest that the oldest mutations associated with lactase persistence reached significant levels in human populations in the last 10,000 years, with the beginning of dairy animal domestication, as this represented human's first contact with ruminant milk. The mutation arose primarily in Northern Europe as theories suggest that due to famine and contamination of water sources, it became evolutionarily beneficial to digest milk. Currently, 35% of adults have this mutation, with higher frequencies in Northern and Central European descendants and in certain African and Middle Eastern populations [1].
Another adaptive mechanism developed by humans due to the nutritional benefits of milk is colonic adaptation in lactose intolerant individuals [8]. Research suggests that a gradual introduction of small amounts of dairy products may trigger adaptation to lactose consumption, due to lactase's prebiotic effect, resulting in a minimization of symptoms.
Lactose-free and lactose-reduced milk, treated with lactase, is nowadays widely available and nutritionally identical to regular milk. Synthetic lactase tablets are also used to attenuate symptoms. Both of these strategies constitute the current cornerstone to overcome lactose intolerance. However, neither of these products have the ability to offer a lactose intolerant individual with a long-term solution to this deficiency and the prospect of adapting to become independent of these resources, in order to enjoy regular dairy again. Instead, they offer a solution based on continual lactase replacement.
Lactose intolerance and potential probiotic treatment
As a possible alternative, the ingestion of probiotics, specifically lactic acid bacteria, by lactose intolerant individuals may mimic the effects of colonic adaptation. By adhering to the intestinal lining and digesting dietary lactose they should alleviate mal absorptive symptoms [1].
A preclinical study has found that Lactobacillus acidophilus supplementation may assist in the breakdown of lactose by lactase-deficient individuals, reducing symptoms in the long term, by altering the intestinal microbiota to create an environment more conducive to the breakdown and absorption of lactose. As the acidophile bacteria reach the small intestine, bile emulsifies the bacterial cell wall. This enables the release of bacterial lactase, which digests any lactose present without leading to the production of gases. Though research is still in its initial stages, bacterial lactase is thought to be superior to synthetic lactase due to its ability to withstand the acidity of the stomach through encasement in the bacterial cells [9]. Additionally, the acidophile bacteria are also capable of absorbing the produced monosaccharides, preventing diarrhea [6]. Therefore, ingestion of probiotics can potentially become the most effective lactose intolerance treatment.
From a group of 38 lactose intolerant individuals, 20 were given a probiotic supplement containing 10 million Colony Forming Units (CFU) of Lactobacillus acidophilus whilst the other 18 received a placebo. After 4 weeks, both groups consumed 480 milli-litres of milk. The group that received probiotics exhibited improved symptoms compared to those who received placebo, including 20.8% less diarrhoea and 18.9% less abdominal cramping [9]. However, studies about which specific probiotic treatment would be more appropriate to aid in the digestion of lactose remains to be determined.
The species of bacteria used are based on commercial availability and on the intention of investigating different genera of bacteria. The three genera of bacteria most commonly used in yoghurt manufacture were selected, considering that it is predictable that previous research have proven their effectiveness in digesting lactose for them to be specifically selected over others [10].
Method
This method was repeated for every species of bacteria, resulting in a total of 5 trials for each species.
Preparation of broth for incubation
In a 100 ml beaker, 0.2 gm of proteose peptone, 0.1 gm of lactose and 0.1 gm of sodium chloride were dissolved in 20 ml of water. A capsule of selected species of bacteria was then incorporated into this broth by mixing.
Incubation of culture
The culture was left incubating inside the beaker in the water bath at 45 degrees Celsius for a total of 24 hours.
Broth for fermentation
In a 1 L beaker, 1 gm of proteose peptone, 1 gm of lactose and 0.5 gm of sodium chloride were dissolved in 100 ml of tap water. This was then separated into five 100 ml beakers, each containing 20 ml of the solution. Each beaker represents a trial.
Calibration of the pH meter
The pH meter was calibrated using buffer solutions of pH 4 and pH 7 before the initial and final measurement of each species. The electrode of the pH meter was cleaned in a saturated potassium chloride solution and wiped dry using tissue paper between each measurement.
Testing
The incubated culture was separated equally into the five beakers containing the broth for fermentation and stirred until homogenous. The five beakers were placed inside the water bath at 45 degrees Celsius, filled with enough water to cover the liquid inside the beaker. The initial pH measurement of the culture was made as soon as the solution was prepared. Measurements were repeated every 45 minutes for a total of 405 minutes. The solution was disposed of in the sink once all measurements were completed.
To ensure reliability of the results collected 1 109 CFU capsules must be used in all trials. The volume of broth for fermentation and for incubation and the quantity of each of its constituent was controlled through precise measurements, ensuring that all colonies are tested with comparable availability of nutrients and environment. The temperature was kept stable, at 45ºC, preventing alteration of the colonies’ metabolic rate. Lastly, the regularity of calibration of the pH meter was controlled so that all measurements made are equally valid.
Error of each species has been calculated accounting for inaccuracy, abbreviated as ‘e. species name’ in the data presented below (Tables 1-3).
| Average pH (± 0.005) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Bacteria species evaluated | ||||||||
| Time (min) | B. therm | e B. therm | B. inf | e B. inf | B. brev | e B. brev | B. long | e B. long |
| 0 | 5.89 | 0.08 | 6.38 | 0.04 | 6.43 | 0.01 | 6.18 | 0.1 |
| 45 | 5.86 | 0.05 | 6.27 | 0.06 | 6.4 | 0.01 | 5.84 | 0.03 |
| 90 | 5.88 | 0.04 | 6.23 | 0.03 | 6.34 | 0.01 | 5.77 | 0.04 |
| 135 | 5.83 | 0.04 | 6.18 | 0.04 | 6.3 | 0.02 | 6.13 | 0.01 |
| 180 | 5.83 | 0.09 | 6.11 | 0.02 | 6.29 | 0.02 | 6.12 | 0.05 |
| 225 | 5.52 | 0.21 | 6.13 | 0.05 | 6.31 | 0.02 | 6.1 | 0.04 |
| 270 | 5.04 | 0.33 | 6.07 | 0.1 | 6.29 | 0.03 | 6.08 | 0.04 |
| 315 | 4.79 | 0.21 | 5.81 | 0.23 | 6.3 | 0.03 | 6.2 | 0.06 |
| 360 | 4.71 | 0.12 | 5.76 | 0.26 | 6.31 | 0.02 | 6.2 | 0.05 |
| 405 | 4.68 | 0.08 | 5.72 | 0.23 | 6.32 | 0.02 | 6.21 | 0.04 |
Table 1: Average change in pH ( ± 0.005) of the medium of Bifidobacterium genus bacteria over a 405-minute period.
| Average pH ( ± 0.005) | ||||||
|---|---|---|---|---|---|---|
| Bacteria species evaluated | ||||||
| Time (min) | L. bulg | e L. bulg | L. casei | e L. casei | L. rham | e L. rham |
| 0 | 6.9 | 0.06 | 6.96 | 0.02 | 7.13 | 0.01 |
| 45 | 6.42 | 0.04 | 5.92 | 0.04 | 6.43 | 0.03 |
| 90 | 6.18 | 0.01 | 5.42 | 0.06 | 6.24 | 0.03 |
| 135 | 5.3 | 0.07 | 5.37 | 0.03 | 6.08 | 0.05 |
| 180 | 5 | 0.08 | 5.25 | 0.07 | 5.94 | 0.08 |
| 225 | 4.98 | 0.04 | 5.12 | 0.09 | 5.89 | 0.08 |
| 270 | 5.04 | 0.07 | 4.97 | 0.15 | 5.93 | 0.09 |
| 315 | 5.07 | 0.05 | 4.78 | 0.17 | 5.98 | 0.12 |
| 360 | 5.05 | 0.13 | 4.56 | 0.24 | 6.23 | 0.18 |
| 405 | 4.98 | 0.18 | 4.41 | 0.21 | 6.41 | 0.22 |
Table 2: Average change in pH ( ± 0.005) of the medium of Lactobacillus genus bacteria over a 405-minute period.
| Average pH (± 0.005) | ||
|---|---|---|
| Bacteria species evaluated | ||
| Time (min) | S. therm | e S. therm |
| 0 | 6.64 | 0.05 |
| 45 | 6.48 | 0.08 |
| 90 | 6.28 | 0.1 |
| 135 | 6.23 | 0.07 |
| 180 | 6.14 | 0.11 |
| 225 | 6.05 | 0.14 |
| 270 | 6.17 | 0.08 |
| 315 | 6.29 | 0.02 |
| 360 | 6.38 | 0.06 |
| 405 | 6.42 | 0.12 |
Table 3: Average change in pH ( ± 0.005) of the medium of Streptococcus genus bacteria over a 405-minute period.
The data presented above shows the average (mean) change in pH of the medium, over a 405-minute period, for each genus of bacteria, comparing distinct species of the same genus. Measurements were made approximately in 45-minute intervals.
The method conducted led to a systematic error due to the pH meter used. It only displayed the pH of the bacterial medium with an accuracy of 2 decimal points. Therefore, the pH recorded could be inaccurate by a value of ± 0.005.
The error value calculated for each species represents the range of the data collected when comparing all trials. Therefore, it is a representation of the random error present in the data collected (Table 4, Figures 1-3).
| Bacterium species | Initial average pH ( ± 0.005) | Final average pH | Total average pH change ( ± 0.005) |
|---|---|---|---|
| ( ± 0.005) | |||
| L. casei | 6.96 | 4.41 | -2.55 |
| L. bulg | 6.9 | 4.98 | -1.92 |
| B. therm | 5.89 | 4.68 | -1.21 |
| L. rham | 7.13 | 6.41 | -0.72 |
| B. inf | 6.38 | 5.72 | -0.66 |
| S. therm | 6.64 | 6.05 | -0.22 |
| B. brev | 6.43 | 6.32 | -0.11 |
| B. long | 6.18 | 6.21 | 0.03 |
Table 4: Ranking of average total pH decrease of culture mediums comparing all species of bacteria tested.
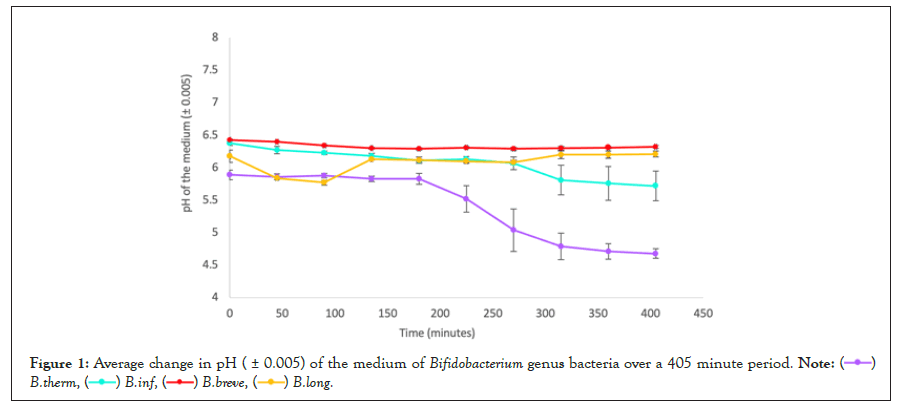
Figure 1: Average change in pH ( ± 0.005) of the medium of Bifidobacterium genus bacteria over a 405 minute period.

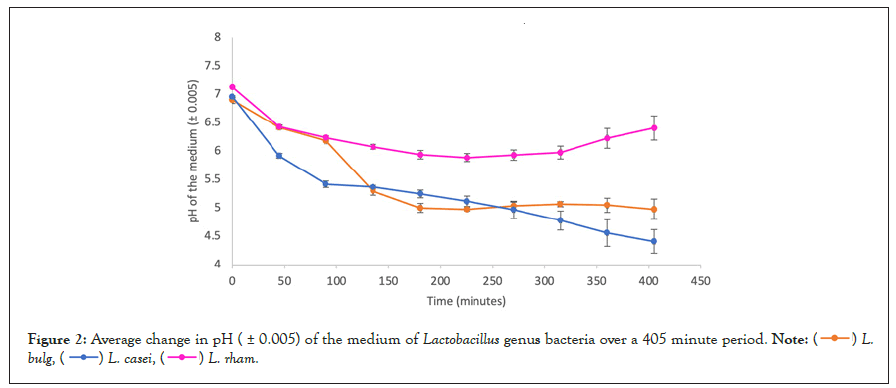
Figure 2: Average change in pH ( ± 0.005) of the medium of Lactobacillus genus bacteria over a 405 minute period.
Figure 3: Average change in pH ( ± 0.005) of the medium of Streptococcus genus bacteria over a 405 minute period.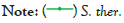
Data analysis
Overall comparison of species effectiveness: Table 4 shows the ranking of average total pH decrease of culture mediums comparing all species 8 species of bacteria tested. The species that led to the most significant pH decrease was L. casei, as the culture medium acidified by 2.55 between the first and last measurements. One species however, B. longum, led to an unexpected positive change in pH. Although it had decreased by 0.41 at the 90-minute mark, it intermittently increased hereafter, leading to an overall pH increase of 0.03 comparing the first and last measurement. All other species led to an overall pH decrease comparing the first and last measurement, ranging from 1.92 to 0.11.
Genera effectiveness: It includes comparison between genera, patterns within each genera and phenomenon of pH increase.
Comparison between genera: Table 4 shows that the specie that caused the second most significant pH decline was L. bulgaricus; overall decrease of 1.92 between the first and last measurement. This indicates that the two species of acidophile bacteria tested that proved most effective at acidifying the medium and thus, at digesting lactose, belong to the genera Lactobacillus spp. The third species of this genera tested, L. rhamnosus, ranked 4th. It proved to be more efficient than S. thermophilus, the only Streptococcus tested, and all the Bifidobacteria tested except for B. thermophilum. Therefore, among the 8 species of bacteria tested, all species of Lactobacilli can be considered more efficient than Streptococcus spp. and L. bulgaricus and L. Casei can also be considered more efficient than all Bifidobacterium species.
Comparing the efficiency of the genera Bifidobacterium and Streptococcus based on the 8 bacteria tested, it is difficult to determine which has higher efficiency at acidifying the medium and thus digesting lactose since 50% of the Bifidobacterium species tested, B. thermophilum and B. infantis experienced greater acidification than S. thermophilus, and the other 50%, B. breve and B. longum, had acidifications inferior to it.
Patterns within each genera: In the data presented in tables 2-4, as well as in figures 1-3, the average pH changes of the medium of the tested species were grouped according to their genera in an attempt to analyse the possible existence of a correlation between bacterial genera and behaviour in a lactose-rich environment, based on whether bacteria in the same genera presented similar pH changes or not.
Analysing figure 1, which presents the pH changes in the medium of 4 different species of Bifidobacteria, it is evident that each species displayed a unique behaviour as shown by the distribution of their data points across the vertical axis, as well as by the changing gradient of the lines that join them. All bacterial culture medium had different initial pH values. The highest starting value was B. breve (6.43), whilst the lowest was B. thermophilum, (5.89). Similarly, the highest final value was B. breve (6.32) and the lowest was B. thermophilum (4.68), displaying a possible correlation between initial and final pH values. However, the opposite correlation is observed when comparing B. infantis and B. longum, as B. longum had a higher initial pH but a lower final one.
In terms of the shape of the pH curve, B. thermophilum and B. infantis, display the most similar behaviours within the genera, as both initially show a period of very minimal pH change, followed by a steep decrease that becomes gradually less steep over time. The B. breve culture experienced a very minimal change in pH over the whole measured period, comparable to the initial behaviour of the two cultures analysed above. However, B. longum had a very distinct behaviour, with a sudden rise in pH after 135, 315 and 405 minutes of exposure. The first two increases were followed by a gradual decrease and the last was recorded on the last measurement so further pH values are unknown. Apart from B. breve, the trend lines representing the different species have 5 a total of 5 points of intersections, 3 of them between B. infantis and B. longum and 2 between B. longum and B. thermophilum. This indicates these culture mediums exhibited identical simultaneous pH values, representative of some degree of similarity in their behaviour.
Figure 2 presents the changes in the medium of 3 different species of Lactobacilli. Once again, although belonging to the same genera, each species behaved distinctly. All initial pH values were different, varying between 6.90 (L. bulgaris) and 7.13 (L. rhamnosus). However, they are more similar than the initial pH values of the Bifidobacteria cultures. Once again, the highest initial pH value is correspondent with the final highest pH value (6.42).
In the case of this genus, different from Bifidobacterium species, all species experienced a decrease in pH when comparing the initial and the final measured value. L. casei and L. rhamnosus display the most significant decrease in pH during the first 45 minutes of incubation. However, the curves of pH change of all species in the genera are extremely distinct overall, with minor similarities.
Comparing L. rhamnosus and L. bulgaricus, an increase in pH was detected at minute 270 and 315. However, while in L. rhamnosus the pH continued increasing until the final measurement, in L. bulgaricus the pH returned to the decreasing trend. The first three data points are similar to each other, almost intersecting. However, after this, the shape of the curves becomes significantly distinct.
In the case of the Streptococcus genera, due to limited commercial availability, only one specie was tested making it impossible to analyse whether there is a trend in behaviour of different species of this genera exposed to such conditions.
Phenomenon of pH increase: As shown by tables 1-4, as well as figures 1-3, the only species that exhibited constant decrease in the medium’s pH was Lactobacillus casei. All other species displayed increase in the medium’s pH in one or more measurement made during the 405-minute fermentation period, although, with the exception of B. longum, there was an overall pH decrease comparing the initial and final measurements.
In B. thermophilum, the pH increased from 5.86 to 5.88 in minute 90 and in B. infantis, from 6.11 to 6.13 in minute 225. The same happened with L. Bulgaricus, which exhibited an increase from pH 5.04 to 5.07 at 315 minutes. However, in these cases, after the fluctuations, the pH change resumed its decreasing trend.
These fluctuations were a lot more prominent in other species. The B. breve culture medium experienced steady pH decrease until minute 180, when it reached a minimum of 6.29. After this, the pH fluctuated between this value and 6.32, increasing in a total of 4 out of 5 measurements. Therefore, the last measurement for this species did not represent the minimum pH reached.
L. rhamnosus and S. thermophilus behaved in very similar ways in terms of pH change, considering that in both cases a decrease in pH was evident until minute 225, when the minimum pH value was reached, 5.89 and 6.05 respectively. After this, the pH values increased steadily. A similar trend is visible for B. longum, although in this case, the minimum pH, 6.08, was reached at 270 minutes, and the increasing trend that followed made the overall change in the pH of the culture positive, +0.03, rather than negative as verified in other species.
When exposed to a lactose-rich environment, the 8 different species of lactic acid bacteria tested displayed distinct acidification of their culture medium presumably due to lactic acid production, indicating varying effectiveness in lactose digestion. The results suggest Lactobacillus Casei to be the most effective specie in digesting lactose, as it achieved the greatest pH decrease and was the only species displayed a constant pH decline.
An unexpected phenomenon was the observed increase in the pH of the culture medium, as the digestion of lactose alone would solely lead to acidification, subsequent to the production of the lactic acid by-product. Although there is no literature exploring this, one could speculate that it is a consequence of some mechanism of bacterial homeostasis manifested to prevent the medium from becoming overly acidic, or atleast to delay acidification, considering that even though lactic acid bacteria are adapted to acidic environments, their metabolic functionality becomes impaired at pH values lower than 4.5. Nevertheless, there is no evidence of such, making it impossible to conclude what these fluctuations in pH suggest about the effectiveness of certain species in digesting lactose. Another possible speculation is related to the fact that one cannot know what possible other constituents were present in the powder form of the bacteria acquired to conduct these experiments and if these unknown substances may have played any role in the pH changes. The pharmaceutical source of the bacteria did not disclose the components used as Quantum Satis Para.
A possible extension to the following research would be to carry out an investigation with the same 8 lactic acid bacteria utilising a method to quantifying change in lactose content as a consequence of the metabolic reactions carried out by the lactic acid bacteria would be a more precise investigation. A method to do so was recently described as a tool to certify lactose- free products, by the addition of an enzymatic assay [11]. This would help conclude whether the pH fluctuations observed are related to the rate and effectiveness of digestion of lactose or not.
The present study provided a simple and effective way to determine whether certain lactic acid bacterial species are more efficient at digesting lactose through fermentation, and therefore possibly more prone to have clinical benefit in probiotic treatment for the lactose intolerant population.
Preclinical studies have been carried out displaying the significance of probiotic treatment with lactic acid bacteria for lactose intolerant individuals to reduce symptoms of flatulence, abdominal pain and diarrhea, presumably due to the effect of bacterial lactase on the small intestine. However, no study had previously described which species of lactic acid bacteria would be most appropriate for this use, to create a more specific and thus effective treatment, therefore inspiring such investigation.
Following investigation, it has been determined that Lactobacillus Casei is the most effective specie in digesting lactose when exposed to a lactose rich environment. This data suggests that this hypothesis is worthy of being explored in a prospective clinical trial designed to evaluate the role of this specie when used as a probiotic supplement for lactose intolerant individuals.
In addition, a recently published small, randomised trial highlighted the potential clinical importance of using probiotic yogurt fortified with Lactobacillus acidophilus and Bifidobacterium species in patients with lactose intolerance as when the hydrogen breath test was applied, the experimental group displayed lower hydrogen levels compared to placebo. This suggests that the lactic acid bacteria in the probiotic effectively digested lactose in the small intestine, thus preventing the process of hydrogen gas production subsequent to the inappropriate digestion of lactose by the naturally occurring microbiome.
The aforementioned randomised clinical trial, although small, constitutes a potential proof of concept that emphasises the importance of further exploring the role of lactic acid bacteria as a potential therapeutic strategy for lactose intolerance. However, our data suggests that Lactobacillus casei is likely to be more effective than the probiotics used by Masoumi et al, therefore justifying further clinical investigation to compare different acidophile bacterial species either isolated or in combination to ensure such treatment achieves its full potential efficacy in this setting.
I am grateful to Leo Novaes for supervising and advising this project.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Katz GF (2022) The Effect of a Lactose-rich Environment on Different Acidophile Bacterial Species. J Prob Health. 10:280.
Received: 01-Sep-2021, Manuscript No. JPH-21-001-Pre-Qc-22; Editor assigned: 06-Sep-2021, Pre QC No. JPH-21-001-Pre-Qc-22 (PQ); Reviewed: 01-Oct-2021, QC No. JPH-21-001-Pre-Qc-22; Revised: 02-May-2022, Manuscript No. JPH-21-001-Pre-Qc-22 (R); Published: 28-Jul-2021 , DOI: 10.35248/2329-8901.22.10.280
Copyright: © 2022 Katz GF. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.