
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Research Article - (2023)Volume 9, Issue 1
Previous studies have revealed that the Receptor-Binding Domain (RBD) of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) spike protein is immunogenic in both mice and healthy volunteers, and humoral immune response plays a key role in RBD-mediated protection. In this study, we investigated the immunogenicity and protective efficacy of immunization with RBD plus three different adjuvants (Al(OH)3, ASO3, and AddaVax) against SARS-CoV-2 pseudo virus through two different routes (intramuscular or intranasal immunization) in mice. The results showed that the immunogenicity and titer of neutralizing antibodies changed in response to different adjuvants and immune routes. Furthermore, six B-cell immunodominant epitopes (RBD1-18, RBD49-66, RBD61-78, RBD97-114, RBD139-156, and RBD145-162) in RBD were identified using antisera from a different immunized group, which may explain the differences in protection observed in each immunized group. Through sequence alignment, we found that six B-cell epitopes are highly conserved among different SARS-COV-2 variants (including Alpha-, Beta-, Gamma- and Omicron-Variant). This study indicated that the immunodominance of epitopes and protection efficacy of RBD antigen are different by different adjuvants and immune routes, which may further guide adjuvant screening for vaccine development and optimization.
SARS-CoV-2; Immunodominant epitope; Antibody; Adjuvants; Neutralizing antibody
RBD: Receptor-Binding Domain; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; ACE2: Angiotensin Converting Enzyme 2; PBS: Phosphate-Buffered Saline; ELISA: Enzyme-Linked Immunosorbent Assay; HRP: Horseradish Peroxidase; SDs: Standard Deviations; PDB: Protein Database; BLAST: Basic Local Alignment Search Tool; RFU: Relative Fluorescence Unit
Although protective isolation and vaccines play crucial roles in controlling disease transmission, more than 333 million cases of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection were confirmed worldwide at the end of October 2022, with nearly 6.55 million deaths (http://covid19.who.int/). To date, more than 10 vaccines have been approved for clinical administration; however, SARS-CoV-2 remains a global health crisis as new variants continue to emerge over time, such as B.1.1.7, B.1.351, B.1.617.2, and B.1.1.529 variants [1-3], Challenging the effectiveness of existing vaccines, Spike (S) protein is one of the major surface glycoproteins of SARS-CoV-2 and the main antigenic component of almost all SARS-CoV-2 vaccines because of its irreplaceable significance in the pathogenesis of the virus [4]. The S protein contains S1 and S2 domains. The S1 domain is involved in virus-host interactions because it contains a Receptor Binding Domain (RBD) that can specifically bind to Angiotensin Converting Enzyme 2 (ACE2) in host target cells, while the S2 subunit participates in viral fusion. Studies have revealed that amino acid mutations in the RBD can lead to changes in the species specificity and susceptibility of the virus [5]. In addition, RBD contains the most immunodominant neutralizing epitope in the S protein, and approximately 90% of neutralizing antibodies in the serum of convalescent patients are induced by this domain [6]. Moreover, the titer of RBD-specific neutralizing antibodies is associated with the clinical outcome of SARS-CoV-2 infection [7] and shows a significant dose-response relationship with protective efficacy [8]. Furthermore, the recombinant RBD-subunit vaccine ZF2001 showed a protective efficacy of 92.93% against the alpha variant and 77.54% against the delta variant in detailed data from the phase 3 clinical trial [9]. Overall, these studies suggested that RBD is the most promising candidate antigen for the development of the SARS-CoV-2 vaccine.
However, the immunogenicity of RBD is limited as it is a small protein with a molecular weight of approximately 32 kDa, and it mainly exists as a monomer in a solution. Thus, it is essential to improve the immunogenicity of RBD for vaccine development. Previous studies have shown that both the vaccination strategy and adjuvants are crucial for enhancing the protective immune response [10], and adjuvants largely affect the immunodominance of epitopes and the protective efficacy of a given antigen [11]. In addition, SARS-CoV-2 infection is typically initiated from the mucosa of the respiratory tract. However, most existing SARS-CoV-2 vaccines are administered intramuscularly, inducing a limited immune response at the site of infection. Therefore, the selection of an appropriate adjuvant, coupled with an alternative immune route, may largely affect the “Immunodominance” [12] of RBD and the host immune response toward the RBD.
In this study, we evaluated the influence of three different adjuvants (Al(OH)3, ASO3, and AddaVax) and two different immune routes (intramuscular immunity and intranasal immunity) in inducing neutralizing antibodies against SARS-CoV-2 pseudovirus. The immunodominance of epitopes and protection efficacy of RBD antigen were differentially altered by different adjuvants and immune routes, wherein we also identified the six novel epitopes of RBD. Our data are of great importance for optimizing the host immune response against RBD and providing strategies for the development of RBD-based SARS-CoV-2 vaccines.
Animals and protein
Six- to eight-week-old specific pathogen-free female BALB/c mice were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Hunan, China). RBD protein was expressed and purified as described previously [13].
Peptide synthesis
18-mer peptides with 12-amino acid length overlapping to cover the full lengths of RBD (Sequence ID: 6XDG_E) were synthesized and purified by GL Biochem Ltd. (Shanghai, China). OVA192-201 (EDTQAMPFRV) peptide was used as a negative control. The purity of these peptides was expected to be 95% or higher. The peptides were dissolved in Dimethyl Sulfoxide at a concentration of 0.5 mg/mL and stored at -80°C before use.
Immunization
To determine the immunogenicity of RBD, mice were randomly assigned into different groups and intramuscularly immunized with 20 µg of RBD combined with 50 µL of AddaVax (InvivoGen, San Diego, CA, USA), AS03 (InvivoGen), or Al(OH)3 (InvivoGen) in a total volume of 100 µL with adjuvant or Phosphate-Buffered Saline (PBS) alone on days 0, 14, and 21. In addition, mice were nasally immunized with the same dose of RBD plus AddaVax on days 0, 14, and 21. One week after the last booster, antisera in each group were collected for the following experiments.
Immunoglobulin subtyping
One week after the last immunization, the mice were exsanguinated, and serum samples were collected. Titers of RBD-specific IgG were determined using an Enzyme-Linked Immunosorbent Assay (ELISA). In summary, microtiter plate wells (Corning, New York, USA) were coated with RBD (0.3 µg per well) in 50 mM carbonate buffer (pH 9.5) at 4°C overnight. Non-specific binding was blocked with 2% Bovine Serum Albumin (BSA; v/v) at 37°C for 2 h. Serum samples were serially diluted twofold in PBS (starting at 1:100) and used as the primary antibodies. Secondary antibodies were Horseradish Peroxidase (HRP) conjugated goat anti-mouse IgG (Sigma, USA) diluted at 1:5000. The absorbance was read at 450 nm (optical density [OD] 450), and the titers were defined as the highest dilution that yielded an absorbance value of more than twice the value of the pre-immune serum. To determine the subtype of RBD-specific IgG, serum samples diluted to 1:1000 were used as primary antibodies, and HRP conjugated goat anti-mouse IgG1, IgG2a, IgG2b and IgG3 (Proteintech, Wuhan, Hubei) were used as secondary antibodies.
ACE2 blocking assay
Antibodies blocking the binding of SARS-CoV-2 spike RBD to ACE2 were detected with an anti-SARS-CoV-2 neutralizing antibody titer serologic assay kit (ACROBiosystems, Beijing, China) according to the manufacturer’s instructions. In brief, serum samples from immunized mice were analyzed in duplicate at a dilution of 1:10 and mixed with 0.3 µg/mL HRP-SARS-CoV-2 spike RBD. The OD value was detected at 450 nm with a micro plate reader, and percentage inhibition was calculated on the basis of the equation (1-average sample OD value/average OD value of negative control) × 100%.
Pseudovirus neutralization assay
The neutralizing antibody of SARS-CoV-2 was quantified using a pseudo typed virus-based assay as previously described [14]. Quantification of SARS-CoV-2 neutralizing antibodies in antisera of RBD-immunized groups was performed by Vazyme Biotech Co., Ltd. For the neutralization assay, pseudo viruses (50 µL) of SARS-CoV-2 (~4 × 105 the relative fluorescence value, RLU) were incubated with serial dilutions of plasma samples (dilutions of 1:50, 100, 200, 400, 800, 1600, 3200, and 6400) from BALB/c mice immunized with RBD+AddaVax (MI), AddaVax (MI), RBD+AddaVax (NI), AddaVax (NI), RBD+AS03 (MI), AS03 (MI), RBD+Al(OH)3 (MI), and Al(OH)3 (MI) for 1 h at 37°C, and then 2 × 104 ACE2-293T cells were added to each cell. Cells without viruses, serum or antibodies served as blind controls, cells with viruses but without plasma or antibodies served as virus controls and cells with Vastand-4 (dilution 1:30, 90, 270, 810, 2430, 7290, 21870 and 65619) were used as positive controls used. Luciferase activities were measured 48 h after infection, and the percentage neutralization was calculated as 100%:(sample signals-blank control signals)/(virus control signals-blank control signals) × 100%. A three-parameter logistical analysis was performed on the full dilution series using Prism 8 (GraphPad Software, San Diego, CA, USA). All data were presented as means ± Standard Deviations (SDs).
Linear B-cell epitope mapping
To determine the reactivity of serum samples from immunized mice against each peptide, microtiter plates were coated with 5 µM of each peptide dissolved in 50 mM carbonate buffer (pH 9.6) and the OVA192-201 peptide was used as a negative control. Non-specific binding was prevented by blocking the coated micro titer plates with PBS (pH7.4) containing 2% BSA. Serum samples from immunized mice (n=10) were diluted in PBS in a 1:50 (v/v) ratio and used as primary antibodies, and rabbit anti-mouse IgG antibodies (Solarbio, Beijing, China) were used as secondary antibodies at a dilution of 1:5000. The ELISA results are shown as absorbance values measured at 450 nm. The normal value for each peptide was calculated by testing sera from naive mouse sera without immunization, and values that were >2.1-fold higher than the mean absorbance value of negative sera was defined as positive.
Structural localization and sequence alignment of the immunodominant epitopes
The crystal structure of RBD (Protein Database (PDB) code: 6M0J) was obtained from the PDB. Immunodominant epitopes were located on these structures using the PyMOL 1.1 program. RBD sequences from different SARS-CoV-2 strains were retrieved from the GenBank database for alignment using NCBI Basic Local Alignment Search Tool (BLAST) software.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.0. All data were presented as means ± SD). Data were analyzed using one-way Analysis Of Variance (ANOVA) with Bonferroni correction. P<0.01 was considered statistically significant.
Host immune response to RBD immunization with different adjuvants
BALB/c mice were immunized with RBD plus different adjuvants by intramuscular injection or intranasal injection on days 0, 14, and 21. The titers and subtypes of RBD-specific IgG in the sera of immunized mice were then measured 7 days after the last booster immunization. As shown in Figures 1 and 2, elevated levels of RBD-specific IgG were detected in all mice immunized with RBD plus adjuvants. In particular, through intramuscular immunization, the highest IgG titer of IgG was noted in mice immunized with RBD plus ASO3 or AddaVax, which was significantly higher than that in mice immunized with RBD plus Al(OH)3. With the same AddaVax adjuvant, a significant difference was observed in RBD-specific IgG titers between intramucsular and intranasal immunization (p<0.0001). Meanwhile, no difference was observed in mice immunized with RBD plus AddaVax and those with RBD plus AS03 (p=0.5342). As shown in Figure 3, analysis of serum IgG subtype in mice immunized with RBD plus different adjuvants revealed a primarily IgG1 response. The levels of subtypes showed a similar trend to the total IgG titers in each group, and no significant differences were observed in the distribution of subtypes between different immunization groups.
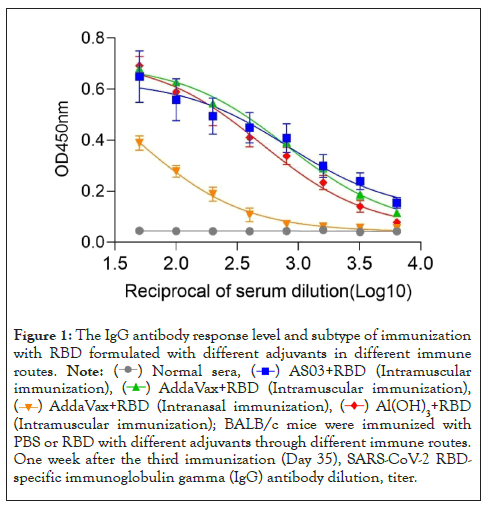
Figure 1: The IgG antibody response level and subtype of immunization
with RBD formulated with different adjuvants in different immune
routes. 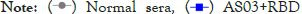 (Intramuscular
immunization),
(Intramuscular
immunization), 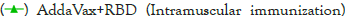 ,
, 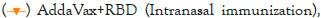
 (Intramuscular immunization); BALB/c mice were immunized with
PBS or RBD with different adjuvants through different immune routes.
One week after the third immunization (Day 35), SARS-CoV-2 RBDspecific
immunoglobulin gamma (IgG) antibody dilution, titer.
(Intramuscular immunization); BALB/c mice were immunized with
PBS or RBD with different adjuvants through different immune routes.
One week after the third immunization (Day 35), SARS-CoV-2 RBDspecific
immunoglobulin gamma (IgG) antibody dilution, titer.
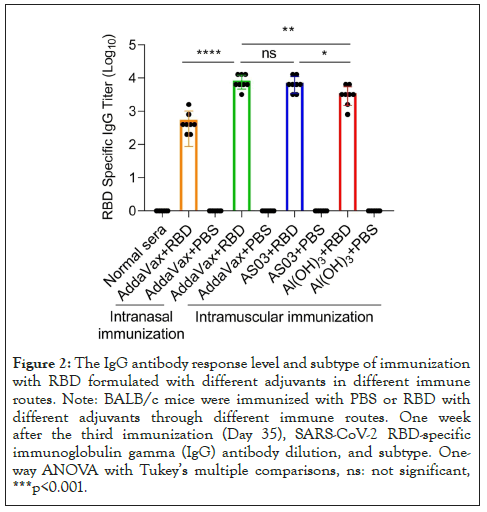
Figure 2: The IgG antibody response level and subtype of immunization with RBD formulated with different adjuvants in different immune routes. Note: BALB/c mice were immunized with PBS or RBD with different adjuvants through different immune routes. One week after the third immunization (Day 35), SARS-CoV-2 RBD-specific immunoglobulin gamma (IgG) antibody dilution, and subtype. Oneway ANOVA with Tukey’s multiple comparisons, ns: not significant, ***p<0.001.
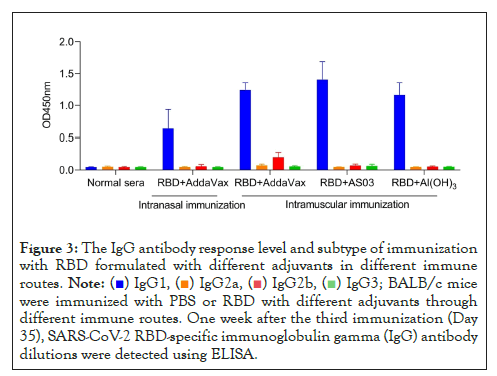
Figure 3: The IgG antibody response level and subtype of immunization
with RBD formulated with different adjuvants in different immune
routes.  BALB/c mice
were immunized with PBS or RBD with different adjuvants through
different immune routes. One week after the third immunization (Day
35), SARS-CoV-2 RBD-specific immunoglobulin gamma (IgG) antibody
dilutions were detected using ELISA.
BALB/c mice
were immunized with PBS or RBD with different adjuvants through
different immune routes. One week after the third immunization (Day
35), SARS-CoV-2 RBD-specific immunoglobulin gamma (IgG) antibody
dilutions were detected using ELISA.
RBD immune serum blocks the binding of SARS-CoV-2 spike RBD to ACE2
To assess the level of functional antibodies induced by RBD plus different adjuvants via different immune pathways, we used a competitive ELISA method to detect neutralizing antibody titers. The PBS group was used as a negative control, and RBD protein alone in intramuscular immunity (MI route) was used as a positive control. Except for the PBS group, the binding of RBD protein to ACE2 increased with increasing serum dilution in the other five groups (Figure 4). Further analysis showed that the 50% neutralization titers induced by RBD in combination with adjuvant were significantly higher than those induced by adjuvant alone (Figure 5). Similar to the levels of RBD-specific IgG, the highest neutralizing antibody titers were observed in mice immunized with RBD plus AddaVax, which were significantly higher than with RBD plus ASO3 or Al(OH)3. With the same AddaVax adjuvant, a significant difference in neutralizing antibody titers was observed between intramuscular and intranasal immunization (NI route) (p=0.0001). The neutralizing antibody titers in mice immunized with RBD plus AddaVax were significantly higher than those of mice immunized with RBD plus AS03 (p=0.0009) or Al(OH)3 (p=0.0005) adjuvants. Meanwhile, the neutralizing antibody titers in mice immunized with RBD plus AS03 and mice immunized with RBD plus Al(OH)3 were not significantly different (p=0.2634).
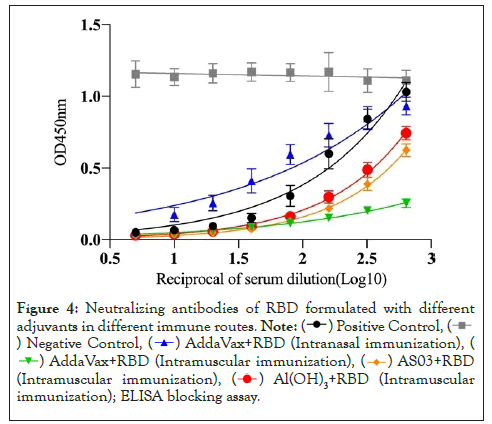
Figure 4: Neutralizing antibodies of RBD formulated with different
adjuvants in different immune routes. 
 (Intranasal immunization), (
(Intranasal immunization), ( (Intramuscular immunization),
(Intramuscular immunization),  (Intramuscular immunization),
(Intramuscular immunization),  RBD (Intramuscular immunization); ELISA blocking assay.
RBD (Intramuscular immunization); ELISA blocking assay.
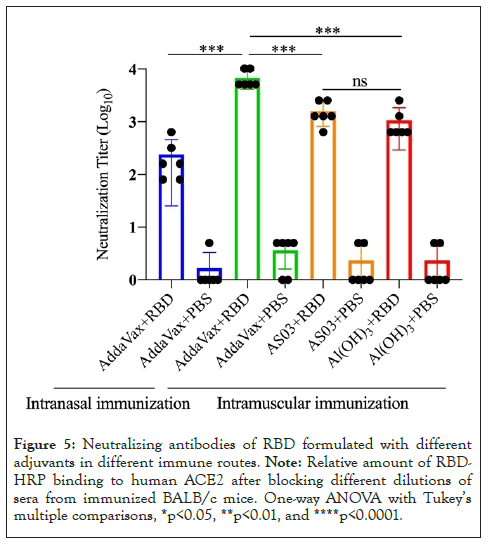
Figure 5: Neutralizing antibodies of RBD formulated with different adjuvants in different immune routes. Note: Relative amount of RBDHRP binding to human ACE2 after blocking different dilutions of sera from immunized BALB/c mice. One-way ANOVA with Tukey’s multiple comparisons, *p<0.05, **p<0.01, and ****p<0.0001.
RBD-mediated protection was altered in response to different adjuvants
We performed a pseudo virus neutralization test in the antiserum of the immune group to RBD and measured the neutralizing antibody titers in the sera of mice in different immunized groups, including 15 BALB/c female mice 6-8 weeks in each group. The sera of mice were acquired after immunization. As expected, after three immunizations, the relative fluorescence value of SARS-COV-2 pseudo virus in mice immunized with 20 µg RBD in AddaVax intramuscular injection (MI) and AS03 intramuscular injection (MI) adjuvants at a dilution of 1:50 was significantly lower than that of SARS-COV-2 pseudo virus in 20 µg RBD in Al(OH)3 adjuvant. The effect of intranasal immunization with AddaVax was not significant (Figures 6 and 7). The Relative Fluorescence Unit (RFU) shows the amount of pseudo virus SARS-COV-2 entering cells; the smaller the value, the stronger the neutralizing antibody. At the same time, the serum of mice immunized with different adjuvants is diluted in a gradient, and the neutralizing titer is measured without adjuvants and in the immunization mode. The results showed that the neutralizing titer of AddaVax (MI) and AS03 (MI) experimental groups is significantly higher than that of their respective control groups (MI), whereas the neutralizing titer of AS03 (MI) is approximately twice that of AddaVax (MI), 1983 and 3211 (Figures 8 and 9). Therefore, we can draw the following conclusion: the serum of mice immunized with AddaVax and AS03, two new adjuvants used in this experiment, had a stronger neutralizing effect on SARS-COV-2 pseudo virus strain than the traditional Al(OH)3 adjuvant, and AS03 (intramuscular injection) had a better neutralizing effect. Furthermore, we studied the effect of AddaVax (MI), AddaVax+RBD (MI), AS03 (MI), and AS03+RBD (MI) on Delta (B.1.617.2) and Omicron (B.1.1.529) mutants and the results showed that AddaVax+RBD (MI) and AS03+RBD (MI) had a cross-protection effect (data not shown).
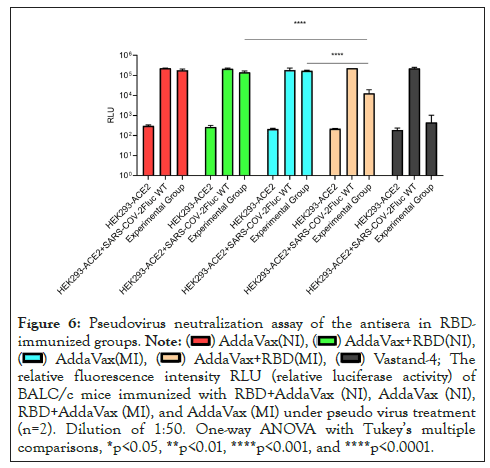
Figure 6: Pseudovirus neutralization assay of the antisera in RBDimmunized
groups. 
 The
relative fluorescence intensity RLU (relative luciferase activity) of
BALC/c mice immunized with RBD+AddaVax (NI), AddaVax (NI),
RBD+AddaVax (MI), and AddaVax (MI) under pseudo virus treatment
(n=2). Dilution of 1:50. One-way ANOVA with Tukey’s multiple
comparisons, *p<0.05, **p<0.01, ****p<0.001, and ****p<0.0001.
The
relative fluorescence intensity RLU (relative luciferase activity) of
BALC/c mice immunized with RBD+AddaVax (NI), AddaVax (NI),
RBD+AddaVax (MI), and AddaVax (MI) under pseudo virus treatment
(n=2). Dilution of 1:50. One-way ANOVA with Tukey’s multiple
comparisons, *p<0.05, **p<0.01, ****p<0.001, and ****p<0.0001.
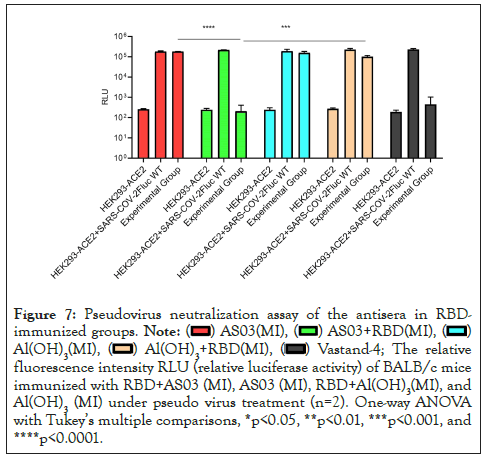
Figure 7: Pseudovirus neutralization assay of the antisera in RBDimmunized
groups. 
 The relative
fluorescence intensity RLU (relative luciferase activity) of BALB/c mice
immunized with RBD+AS03 (MI), AS03 (MI), RBD+Al(OH)3(MI), and
Al(OH)3(MI) under pseudo virus treatment (n=2). One-way ANOVA
with Tukey’s multiple comparisons, *p<0.05, **p<0.01, ***p<0.001, and
****p<0.0001.
The relative
fluorescence intensity RLU (relative luciferase activity) of BALB/c mice
immunized with RBD+AS03 (MI), AS03 (MI), RBD+Al(OH)3(MI), and
Al(OH)3(MI) under pseudo virus treatment (n=2). One-way ANOVA
with Tukey’s multiple comparisons, *p<0.05, **p<0.01, ***p<0.001, and
****p<0.0001.
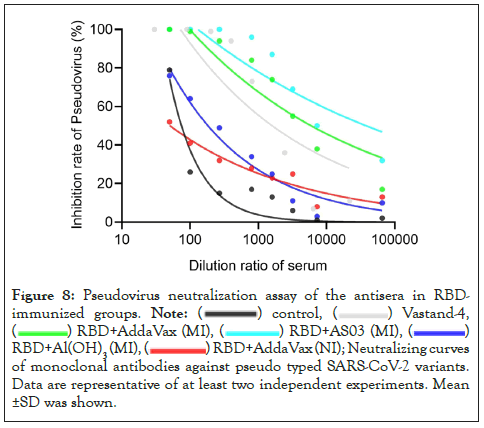
Figure 8: Pseudovirus neutralization assay of the antisera in RBDimmunized
groups. 
 RBD+Al(OH)3(MI),
RBD+Al(OH)3(MI),  Neutralizing curves
of monoclonal antibodies against pseudo typed SARS-CoV-2 variants.
Data are representative of at least two independent experiments. Mean
±SD was shown.
Neutralizing curves
of monoclonal antibodies against pseudo typed SARS-CoV-2 variants.
Data are representative of at least two independent experiments. Mean
±SD was shown.
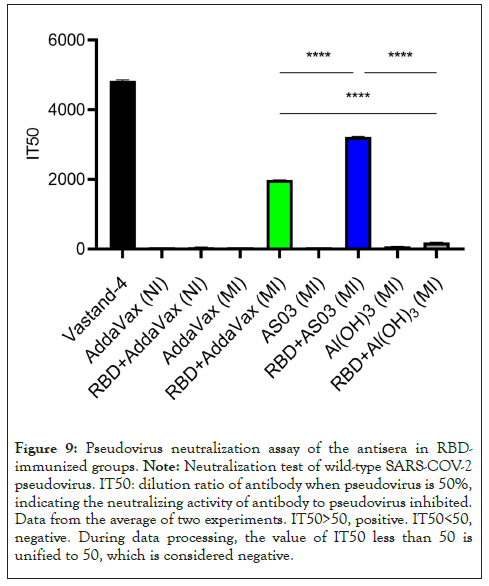
Figure 9: Pseudovirus neutralization assay of the antisera in RBDimmunized groups. Note: Neutralization test of wild-type SARS-COV-2 pseudovirus. IT50: dilution ratio of antibody when pseudovirus is 50%, indicating the neutralizing activity of antibody to pseudovirus inhibited. Data from the average of two experiments. IT50>50, positive. IT50<50, negative. During data processing, the value of IT50 less than 50 is unified to 50, which is considered negative.
Identification of immunodominant epitopes on RBD using serum from mice immunized with RBD plus different adjuvants
Linear B-cell epitope mapping was performed by ELISA using overlapping 18-mer peptides and antiserum from mice immunized with RBD and different adjuvants by intramuscular or nasal immunization. Six epitopes of RBD were identified to have strong IgG reactivity in Figure 10, and the sequences of these epitopes are listed in Table 1. Among these immunodominant epitopes, RBD145-162 was immunodominant in the RBD plus Al(OH)3, AddaVax, and AS03 groups. RBD1-18 was only immunodominant in the RBD plus AS03 group, while RBD49-66 was only immunodominant in the RBD plus Al(OH)3 group. The epitopes RBD1-18, RBD49-66, RBD139-156, and RBD145-162 were immunodominant in the intramuscular immunity group, while RBD61-78 and RBD97-114 were immunodominant in the intranasal immunity group. These results suggested that both adjuvant immune routes could drive an immunodominant response toward the same antigen. Meanwhile, the immunodominant epitope RBD145-162 identified can share the same B-cell epitope in RBD152-160 (TEIYQAGST). The same was previously identified using antisera from BALB/c mice that were immunized with RBD-mFc protein formulated with aluminum hydroxide adjuvant and CpG oligonucleotides [15]. A previous study identified a B-cell epitope in RBD122-142 (NLDSKVGGNYNYLYRLFRKSN) using antisera from C57BL/6 mice immunized with CRM197-RBD peptide conjugates [16], which was not identified in this study. We thought that cross-reactive material (CRM197) might remodel the immunodominance of the antigen. Essentially, the five epitopes RBD1-18, RBD49-66, RBD61-78, RBD97-114, and RBD139-156 have not been previously reported and, therefore, may harbor novel linear B-cell epitopes.
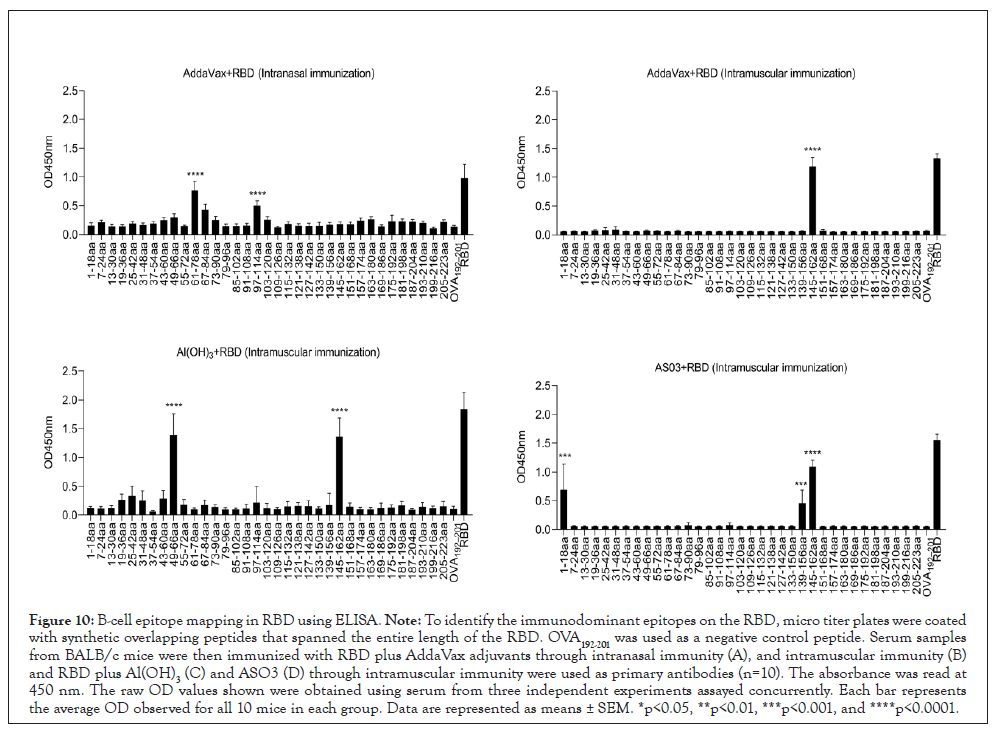
Figure 10: B-cell epitope mapping in RBD using ELISA. Note: To identify the immunodominant epitopes on the RBD, micro titer plates were coated with synthetic overlapping peptides that spanned the entire length of the RBD. OVA192-201 was used as a negative control peptide. Serum samples from BALB/c mice were then immunized with RBD plus AddaVax adjuvants through intranasal immunity (A), and intramuscular immunity (B) and RBD plus Al(OH)3(C) and ASO3 (D) through intramuscular immunity were used as primary antibodies (n=10). The absorbance was read at 450 nm. The raw OD values shown were obtained using serum from three independent experiments assayed concurrently. Each bar represents the average OD observed for all 10 mice in each group. Data are represented as means ± SEM. *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
| The immunodominant epitope | Adjuvant | Sequence of the immunodominant epitope | Immunization routes |
|---|---|---|---|
| RBD1-18 | AS03 | RVQPTESIVRFPNITNLC | Intramuscular immunity |
| RBD49-66 | Al(OH)3 | VLYNSASFSTFKCYGVSP | Intramuscular immunity |
| RBD61-78 | AddaVax | CYGVSPTKLNDLCFTNVY | Intranasal immunity |
| RBD97-114 | AddaVax | TGKIADYNYKLPDDFTGC | Intranasal immunity |
| RBD139-156 | AS03 | RKSNLKPFERDISTEIYQ | Intramuscular immunity |
| RBD145-162 | AddaVax, Al(OH)3, AS03 | PFERDISTEIYQAGSTPC | Intramuscular immunity |
Table 1: Sequences of the immunodominant epitopes on the RBD identified in this study.
Localization and sequence alignment of the immunodominant epitopes on RBD
As the crystal structures of RBD are available in the PDB, we then located the seven epitopes on the structure of these proteins. All three epitopes are located on the surface of these structures, making them more accessible to specific antibodies (Figure 11). Among these epitopes, RBD139-156, RBD145-162, and RBD1-18 assemble into α-helix structures. In contrast, the other epitopes RBD49-78 and RBD97-114 assemble into a complex of loop and α-helix structures.
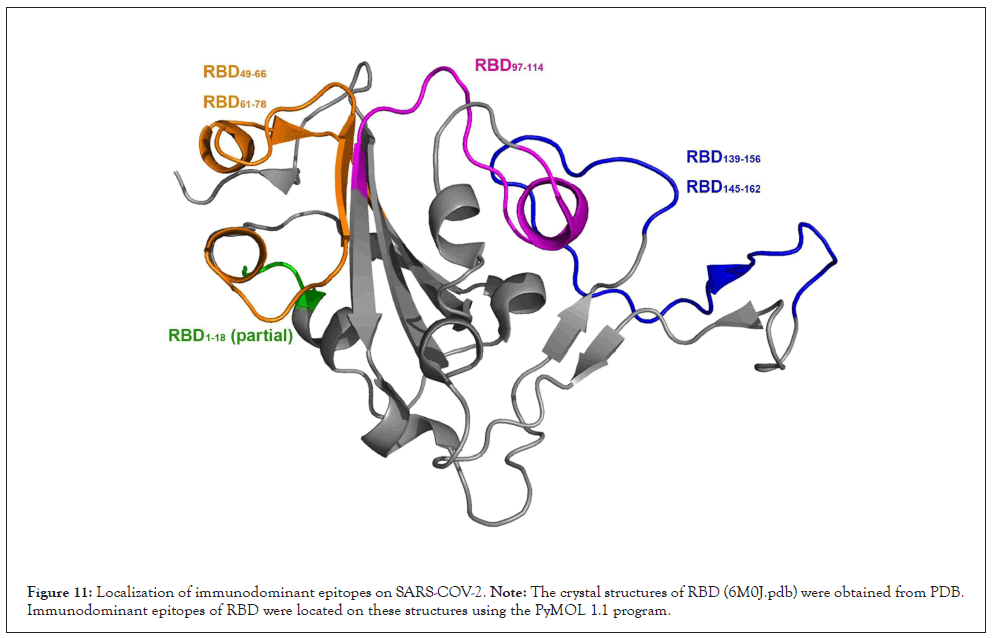
Figure 11: Localization of immunodominant epitopes on SARS-COV-2. Note: The crystal structures of RBD (6M0J.pdb) were obtained from PDB. Immunodominant epitopes of RBD were located on these structures using the PyMOL 1.1 program.
To determine the conservation of these immunodominant epitopes, the amino acid sequences of RBD from alpha, beta, gamma, delta, and omicron randomly selected SARS-CoV-2 strains were retrieved from the GenBank database for alignment. The results revealed that sequences of all six immunodominant epitopes identified in this study were highly conserved among these SARS-CoV-2 strains, with amino acid identities of approximately 99.5% (Table 2). Therefore, specific antibodies targeting these epitopes may cross-react with different variants of SARS-CoV-2.
| # Virus Strain | RBD1-18 | RBD49-66 | RBD61-78 | RBD97-114 | RBD139-156 | RBD145-162 |
|---|---|---|---|---|---|---|
| BCN86353 | RVQPTESIVRFPNITNLC | VLYNSASFSTFKCYGVSP | CYGVSPTKLNDLCFTNVY | TGKIADYNYKLPDDFTGC | RKSNLKPFERDISTEIYQ | PFERDISTEIYQAGSTPC |
| Wild-type | ||||||
| UHТ90657 | RVQPTESIVRFPNITNLC | VLYNSASFSTFKCYGVSP | CYGVSPTKLNDLCFTNVY | TGKIADYNYKLPDDFTGC | RKSNLKPFERDISTEIYQ | PFERDISTEIYQAGSTPC |
| Alpha-Variant | ||||||
| UJB55404 | RVQPTESIVRFPNITNLC | VLYNSASFSTFKCYGVSP | CYGVSPTKLNDLCFTNVY | TGNIADYNYKLPDDFTGC | RKSNLKPFERDISTEIYQ | PFERDISTEIYQAGSTPC |
| Beta-Variant | ||||||
| UIK22186 | RVQPTESIVRFPNITNLC | VLYNSASFSTFKCYGVSP | CYGVSPTKLNDLCFTNVY | TGKIADYNYKLPDDFTGC | RKSNLKPFERDISTEIYQ | PFERDISTEIYQAGSTPC |
| Gamma-Variant | ||||||
| UJO50681 | RVQPTESIVRFPNITNLC | VLYNSASFSTFKCYGVSP | CYGVSPTKLNDLCFTNVY | TGKIADYNYKLPDDFTGC | RKSNLKPFERDISTEIYQ | PFERDISTEIYQAGSTPC |
| Delta-Variant | ||||||
| UTZ18954 | RVQPTESIVRFPNITNLC | VLYNFAPFFAFKCYGVSP | CYGVSPTKLNDLCFTNVY | TGNIADYNYKLPDDFTGC | RKSNLKPFERDISTEIYQ | PFERDISTEIYQAGNTPC |
| Omicron-Variant |
Note: The sequences of SARS-COV-2 wild-type and Alpha, Beta, Gamma, Delta, and Omicron mutants were retrieved from the GenBank database. These sequences were aligned using the NCBI BLAST software.
Table 2: Sequence alignment of the immunodominant epitopes on different SARS-COV-2 strains.
The coronavirus disease 2019 (COVID-19) caused by the novel SARS-CoV-2 has resulted in economic losses and threatened human health worldwide. Because of the high morbidity and mortality associated with SARS-CoV-2 infections and the increased infection rate, vaccination is the most valuable and cost-effective health measure to prevent and control COVID-19 [3]. To date, several SARS-CoV-2 vaccines have been developed [17]. Clinical trials have demonstrated that the spike protein was immunogenic and well tolerated in healthy volunteers, and it induced a robust and RBD-specific immune response [18]. In addition, previous studies have shown that antibody levels of RBD are positively associated with the survival of patients with COVID-19 [19]. From the view of neutralizing activity, RBD is the most immunogenic antigen in the S1 domain of the spike protein of SARS-CoV-2 and binds to ACE2, which mediates the entry of SARS-CoV-2 and is also closely associated with the pathogenesis of COVID-19 [20]. Passive immunization with anti-SARS-CoV-2 antibodies through human convalescent plasma appears to be a very promising approach for protracted COVID-19 symptoms in patients unable to mount a specific humoral response against SARS-CoV-2 [21].
The co-administration of adjuvants is crucial for the protective immune response [10]; thus, it is reasonable to use adjuvants to assist the effectiveness of vaccines and overcome potential vaccine shortages. Appropriately selected adjuvant technologies can decrease the amount of virus vaccine antigen required per dose and may broaden or lengthen the conferred protection against the disease. During the past two decades, several adjuvants have been developed, including aluminum salt (Alum) adjuvants [22], AS03 [23], and AddaVax [24]. Alum is commonly used to slow the release of antigens from immune sites [25]. Alum was used as an adjuvant in SARS-CoV-2 vaccines, including RBD protein vaccines and inactivated vaccines. Both are currently under clinical trials [26,27]. A previous study showed that an aluminum adjuvant-assisted SARS-COV-2 subunit vaccine has a Th2-oriented response [28]. AddaVax is an oil-in-water Nano emulsion that can induce high levels of neutralizing antibodies and produce a strong Th1-biased immune response in COVID-19 vaccines [29]. AS03 is an oil-in-water emulsion adjuvant similar to MF59 [30]. AS03 enhances the antibody response and CD4+ T-cell response and is licensed for pandemic influenza vaccines [31]. In this study, Al(OH)3, AddaVax, and AS03 adjuvants proved to be efficient in inducing humoral immunity against RBD. However, the differences and mechanisms of these adjuvants in stimulating protective immunity in vaccines against RBD remain unknown. In addition, no reports exist about the mechanism of these adjuvants used in the different immune routes against SARS-CoV-2.
Vaccine efficacy is attributed to the robust production of protective antibodies against protein antigens. In this study, RBD-mediated protection changed in response to different adjuvants. Mice were immunized with RBD formulated with different adjuvants, and the titers of RBD-specific IgG, IgG subtype, and B-cell linear epitopes in immunized mice were further analyzed. Mice immunized with RBD plus different adjuvants of SARS-CoV-2 showed significant differences in response to RBD immunization, and the titers of the immunization groups varied. As expected, the RBD-specific IgG titers were closely associated with the neutralizing capacities of ACE2 in each group, consistent with previous findings that the humoral immune response is essential for SARS-CoV-2-mediated protection [32]. Furthermore, the analysis of the serum IgG subtype in mice immunized with RBD plus different adjuvants revealed a primary IgG1 response. A recent study compared different available adjuvants, including alum and AddaVax, by combining them with plant-produced RBD-Fc, in which both adjuvants induced a high level of total IgG and neutralizing antibodies. Furthermore, the adjuvants tested significantly enhanced IgG1 response levels in mice [33]. This is consistent with our experimental results.
The protective response induced by an antigen is largely mediated by its immunodominant epitopes [34], which has been shown in various viral infections, including influenza virus [35], porcine epidemic diarrhea virus [36], and SARS-CoV-2 [37]. Immune responses induced by different immunodominant epitopes of the same antigen can result in different protective efficacies [38]. The results showed that RBD-mediated protection changed in response to different adjuvants and immune routes. Furthermore, six B-cell immunodominant epitopes were identified in the RBD, including four novel epitopes in intramuscular immunity and two novel epitopes in intranasal immunity. Among these immunodominant epitopes, RBD145-162 was immunodominant in the RBD plus Al(OH)3, AddaVax, and AS03 groups. RBD1-18 was only immunodominant in the RBD plus AS03 group, while RBD49-66 was only immunodominant in the RBD plus Al(OH)3 group. The mapping of B-cell epitopes revealed different immunodominant epitopes, indicating that the difference in protective immunity observed in different immunization groups may be due to the different antibody titers induced in response to different adjuvants or route-dependent epitope specificities. Sequence alignment indicated that all epitopes were conserved between different isolates of SARS-CoV-2, indicating that antibodies recognizing these epitopes can provide broad protection against infections caused by different isolates of SARS-CoV-2 [39].
In summary, five novel epitopes were identified using serum from mice immunized with RBD plus different adjuvants through different immune routes. This study showed that RBD-mediated protection and immunodominance were modified in response to different adjuvants, and RBD-mediated protection even changed with the same adjuvant in different immune routes. This study may shed light on the molecular mechanisms of RBD-mediated protection against SARS-CoV-2 infection. It is crucial to select the appropriate adjuvant and immune route to optimize the protective efficacy of the SARS-CoV-2 vaccine as the adjuvants Al(OH)3, AddaVax, and AS03 plus the same RBD induced different immunodominant B-cell responses. In the future, CD4+ and CD8+ T-cell responses in RBD immunization with different adjuvants need to be investigated.
In conclusion, this study showed that RBD-mediated protection changed in response to different adjuvants, and it changed even with the same adjuvant in different immune routes. Six B-cell immunodominant epitopes on RBD were identified, which included four novel epitopes (RBD1-18, RBD49-66, RBD139-156, and RBD145-162) in the intramuscular immunity and two novel epitopes (RBD61-78 and RBD97-114) in the intranasal immunity. In addition, the B-cell immunodominant epitopes identified from mice immunized with RBD plus different adjuvants were different from each other, which may explain the difference in protective immunity observed in each immunized group. Thus, the results showed that adjuvants mainly and immune routes affected the immunodominance of epitopes and the protective efficacy of RBD, which may further guide adjuvant screening for vaccine development and optimization.
All animal experiments carried out in this study were approved by the Animal Ethical and Experimental Committee of the Third Military Medical University (Chongqing, Permit No. SCXK 20170004).
Not applicable.
Not applicable.
The authors declare that they have no competing interests.
This work was supported by grants from the National Natural Science Foundation of China (No.31970869 and 31970138) and the Natural Science Foundation Project of Chongqing (cstc2020jcyjmsxmX0353).
Zhuo Zhao, Wanneng Wang, Ni Li designed research. SisiLi, Lianli Duan, Xiaoli Zhang, Rui yang, Longlong Chen, Zhifu Chen, Qiang Gou, Wenxin Bao, Yue Yuan, and Haiming Jing performed the experiments. Zhuo Zhao, SisiLi, Lianli Duan, Xiaoli Zhang and Rui yang analyzed the data. Yi zhang, Ping Chen and Ping Luo contributed reagents/materials/analysis tools. Zhuo Zhao, SisiLi, and Lianli Duan wrote the paper.
We thank all the researchers who have uploaded and shared their databases to make this work possible.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Li S, Duan L, Zhang X, Yang R, Chen L, Chen Z, et al. (2023) The Effect of Adjuvants and Immune Routes on the Immunodominance of Epitopes and Protection Efficacy of SARS-CoV-2 RBD Antigen. Immunotherapy (Los Angel). 9:211.
Received: 05-Dec-2022, Manuscript No. IMT-22-20627; Editor assigned: 08-Dec-2022, Pre QC No. IMT-22-20627 (PQ); Reviewed: 27-Dec-2022, QC No. IMT-22-20627; Revised: 03-Jan-2023, Manuscript No. IMT-22-20627 (R); Published: 10-Jan-2023 , DOI: 10.35248/2471-9552.23.09.211
Copyright: © 2023 Li S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.