Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2015) Volume 5, Issue 4
The effect of pH on foaming properties on cowpea (Vigna ungiculata) seeds as whole cowpea flour (WCF), dehulled cowpea flour (DCF), dehulled defatted cowpea flour (DDCF) and cowpea protein isolates (CPI) obtained from DDCF by isoelectric (CPIA) and micellization (CPIB) precipitation was studied. The protein % was found to be 22.3% in (WCF) and 26% in (DDCF) flour, while 75 and 76% for CPIA and CPIB respectively. The foaming capacity (FC) and foam stability (FS) were greatly affected by pH values. FC of DDCF and CPIB showed minimum level at pH 2.0 while CPIA at pH 4.0. However, the FS of DDCF was highest at pH 5.0 while CPIA and CPIB were less stable. CPIB have poor FS compared to CPIA at all pH ranges (2-12). Maximum FS was observed at pH 9.0 for DDCF which remain stable up to standing period of 2 hr. CPIA form foam with highest initial volume and maximum FS was observed at pH 9.0. FC of all products was found to be higher at alkaline region of pH compared to acidic side. Defattening markedly increase the FC in the flours.
Keywords: Whole cowpea seed, De-hulled cowpea seed, Isoelectric protein isolate, Micellization protein isolate, Foaming properties
Plant proteins play significant role in human diet especially in developing countries where average protein intake is less than required [1]. In Sudan, as in most tropical countries little work has been carried out either on composition or cultivation of legume crops. The proteins in cereals and millets which form the major component of the poor man’s diet are deficient in lysine ant that they can be supplemented by legumes which are rich not only in lysine but also in threonine [2,3]. Cowpea (Vigna ungiculata L. Walp) is widely cultivated and consumed in Africa and South America. It provides more than half the plant protein in human diets for the poorest sector of many developing countries. It is an important source of protein in developing countries, especially in West Africa where it is eaten in a variety of ways [4]. Okaka and Potter, [5] showed that a blend of 90% wheat flour with 10% drum dried cowpea powder produced excellent quality yeast bread. McWatters [6] prepared cowpea flour which replaced the milk protein in a chemically leavened quick bread (biscuits). The amount of protein in cowpea varies with cultivars, ranged from 18.3%−30%. Nugdalla and Eltinay, [7] Ragab et al., [8] showed that the protein content of cowpea seed was found to be 26.8%. Legumes protein isolates or concentrates have gained importance in the food industry because of their high protein content. They provide nutritional quality due to lower anti nutritional factors with minimum off odour and colour [9]. The concentrate and isolate forms of protein are widely used as an ingredient due to their functional properties; which can be optimized by knowing the chemical and physicochemical characteristics of these proteins. The production of plant protein isolates is of growing interest to industry because of the increasing application of plant proteins in food and non-food markets. The use of plant isolates in foods as functional ingredients, to improve the nutritional quality of the product or for economic reasons is much extended. Foam capacity is usually expressed as percentage volume increase. Lawhon et al., [10] reported that the volume of foam at 30 sec as foam capacity. The foam stability refers to the ability of protein to stabilize against gravitational and mechanical stresses. Foaming properties include whip ability and foam ability; both terms are used interchangeably by various researchers. Hettarachchy and Ziegler, [11] also reported that foam stability is affected by rheological properties such as viscosity and shear resistant, film elasticity, and the magnitude of disjoining pressure between the protein layers. The effect of pH on whey protein isolate (WPI) was studied, pH 5 showed highest over run values and highest FS than those formed at pH 7.0 or 4.0 [12]. The objective of this study was to prepare two types of protein isolates from dehulled cowpea seed flour using the isoelectric precipitation, (CPIA) and micellization precipitation (CPIB), and to study and the influence of pH on foaming properties of (DDCF), (CPIA and CPIB).
Samples
Seeds and Dehulled cowpea (Vigna unguiculata L.walp) of white colored seed (Figure 1) were brought from the local market at Wad Medani city, Sudan, and stored in polyethylene bags at room temperature (29°C−30°C).
Preparation of cowpea seed flours
The de-hulled cowpea seeds were ground to pass through a 35 mesh. The flour was defatted by soaking in petroleum ether at room temperature for 48 hr. with several changes of the solvent. The solvent was decanted and the defatted flour was air dried over night at room temperature and kept in clean closed bottles ready for analysis.
Protein isolates-A, (CPIA)
Cowpea protein isolate A (CPIA) was prepared as shown in Figure 2 following the isoelectric method described by Thompson, slightly modified by Mccurdy and Knipfel, [13], Fernandez et al. [9]. The isolate was freeze dried; ground into powder using a ceramic mortar and pestle and stored in desiccators.
Protein isolate-B, (CPIB)
Cowpea protein isolate B (CPIBA) was prepared as shown in Figure 2 using micellization method. The isolate was freeze dried; ground into powder using a ceramic mortar and pestle and stored in desiccators.
Foam capacity (FC) and foam stability (FS)
FC and FS at different pH levels were determined by the method of Lawhon et al., [10] with slight modification. DDCF, CPIA fo, and CPIB, 1 g each were whipped with 100 ml distilled water for 5 min at the highest speed at room temperature.
The contents along with foam were immediately poured into a 250 ml measuring cylinder. The volume of foam (ml) at 30 seconds was calculated, and the volume increase is expressed as % FC.
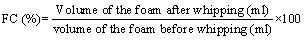
Foam stability (FS)
Determined by measuring the decrease in volume of foam as a function of a time up to period of 120 min, the stable foam volumes were recorded at time intervals of 5, 10, 15, 20, 30, 40, 50, 60, 90 and 120 min
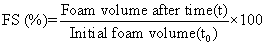
Where: t0 = the starting time immediately after blending.
t= the time at which the foam volume is increase.
The FC and FS at room temperature were determined as the function of pH of the products (DDCF, CPIA and CPIB). The pH was adjusted to desired value (2, 4, 7, 9, and 12) with either 1 or 0.1 N NaOH prior to whipping
Statistical analyses
Data represent means of triplicate samples, which were subjected to analysis of variance and means were separated according to using Duncan multiple range test [14,15]. The significant difference was determined at 0.05 levels.
Proximate composition of the seed flour and the protein isolates are presented in Table 1. The data obtained are comparable to that reported by Abdalla et al., [16], Ragab et al., [8] Sosulski et al., [17]. CPIA and CPIB showed 75% and 76% protein content and a decrease in carbohydrate content to 13% (Table 1).
Table 1: Proximate composition (%) of whole cowpea flour (WCF), de-hulled defatted cowpea flour (DDCF) and protein isolates (CPIA) and (CPIB) (on dry basis). Means in the same raw with different letters are significantly different (P<0.05). Means ± standard deviation of triplicate an LSD = Least significant differences.
Foaming capacity and stability
FC of cowpea flour and protein isolates (Figure 3) was pH dependent and higher at alkaline region of pH compared to acidic side. FCs of DDCF, CPIA and CPIB showed minimum level at pH 9.0, 4.0 and 2.0 respectively. At pH 12.0 CPIA exhibited highest foam capacities. However, FS of DDCF was highest at pH 5 while CPIA and CPIB were less stable. CPIB have poor FS compared to CPIA at all pH range. Maximum FS was observed at pH 9.0 for DDCF which remain stable up to standing period of 2 hr (Table 2). CPIA showed highest initial foam volume and maximum FS at pH 9.0 (Table 2).
Table 2: Effect of pH on foam capacity and stability of dehulled defatted cowpea flour (DDCF) and cowpea protein isolates (CPIA and CPIB) SEM ± is standard error mean. Means in the same column followed by the same letter (s) are not significantly different according to Duncan’s Multiple Range Test (DMRT) C.V % coefficient of variance.
The foam property of a product was found to be important and FS is the most important factor. The effect of pH on FC of DDCF, CPIA and CPIB at different pH values are shown in Table 2.
Maximum values in foam volume were at pH 12.0: 130%, 170% and 100% for DDCF, CPIA and CPIB respectively (Figure 3). The higher FC at pH 12.0 was likely due to increase of the net charges on the protein, which weakened the hydrophobic interactions but increased the flexibility of the protein. This allowed the protein to diffuse more rapidly to the air–water interfaces to encapsulate air particles and then to enhance the foam formation due to increase in protein solubility at alkaline pH [18].
Results of FC for DDCF, CPIA and CPIB was lower than those reported for soy bean protein concentrate and isolate by Sosulski and Feleming, (1977); mung bean flour and cowpea protein isolates, (CPIA and CPIB) by Coffman and Garcia, (1977). Foaming was concentration dependent and increased with increasing of the protein in the aqueous dispersion. However, FC recorded for DDCF (53%) at pH 7.0 (Figure 3); is higher than those reported for cowpea flour, (40%) as reported by Abbey and Ibeh, [19] and pumpkin seed flours (13.2%) [20]. De-fatting markedly increases the foam capacity in the flours. It was reported that foam ability is related to the rate of decrease of the surface tension of the air/water interface caused by absorption of protein molecules [21].
FC of DDCF at neutral pH (pH 7.0) was higher than that for cowpea protein isolates (CPIA and CPIB) Figure 3. Giami, [22]; Naryana and Narsinga-Rao, [23] reported that heat processing considerably decreased the FC and FS of cowpea flour and winged bean flour. The foam of flours has been shown to be related to the amount of native protein [24]. The foam properties of cowpea flour has been shown to be a desirable characteristics for the production of several cowpea based food products [25]. The foam capacity of DDCF, CPIA and CPIB was found to be higher at alkaline region of pH compared to acidic side Figure 3. This may be due to strong forces between protein molecules which prevent the unfolding and spreading of protein molecules. Similar observations have bean reported for soy protein isolate, caseinate and whey protein concentrate [26] and cotton seed protein isolate [27]. The effect of pH on FS of (DDCF) and both protein isolates (CPIA and CPIB) is shown in Figures 4a, b and c respectively. DDCF had the highest initial foam volume (95.0 ml) at pH 2.0. The foam was very unstable compared with that at other pH values (Figure 4a), the foam at this pH showed a very sharp and rapid decrease in foam volume up to standing period of 2 hr.
In fact maximum FS was observed at pH 9.0 which remain stable (90 ml) up to standing period of 2 hr. The high stability of foams in alkaline pH may have been due to the formation of stable molecular layers in the air-water interface, which impact texture, stability, elasticity to the foams. Poor foaming properties were found at pH 4.0; this may be due to the decrease in the net charge of the protein molecules, at the isoeletric points, reducing electrostatic repulsion. Liao and Mangino, [28] showed that foam formation depends upon the solubility of the proteins which is related to their hydropholicity
In fact maximum FS was observed at pH 9.0 which remain stable (90 ml) up to standing period of 2 hr. The high stability of foams in alkaline pH may have been due to the formation of stable molecular layers in the air-water interface, which impact texture, stability, elasticity to the foams. Poor foaming properties were found at pH 4.0; this may be due to the decrease in the net charge of the protein molecules, at the isoeletric points, reducing electrostatic repulsion. Liao and Mangino, [28] showed that foam formation depends upon the solubility of the proteins which is related to their hydropholicity.
Like DDCF, CPIA, formed a foam with highest initial volume (92.0 ml) and maximum foam stability was observed at pH 9.0 (Figure 4b). The foam at this pH showed gradual decrease in foam volume up to standing period 2hr. The foam of CPIA at pH 7.0 had the least initial Volume (72 ml) compared with foams of other pH values. Moreover its FS was comparable to the foams of pH 9.0 which showed collectively, better stability than the foam of pH 2.0, 4.0 and 12.0 with a narrow difference in between their foam volumes during the 2h standing period [29-32]. The high FS for CPIA was obtained at pH 9.0 since this foam decreased gradually at a moderate rate over two hours. Poor FS was observed at pH 12.0 which showed approximately least volume almost in all times (Figure 4c).
With regard to CPIB, poor stabilities were observed at pH 12.0. The foam at this pH showed least volumes, zero value all the time periods tested (5, 10, 20 30, 40, 50, 60, 90 and 120 minutes). However, good foaming properties with highest stability of foams in acid pH range was observed in CPIB, in the present investigation may have been due to the formation of stable molecular layer in air-water interface, which impact texture, stability and elasticity to the foams. Poor foam properties was formed at pH 12.0 for CPIB which showed least volume almost in all times and completely collapsed after first 5 minutes period of their whipping, whereas non of other samples was completely collapsed.
Protein isolates (CPIA and CPIB) showed 75% and 76% protein content and carbohydrate content decrease from 59.78% in DDCF to 13% in protein isolates. The foam capacity (FC) of cowpea flour and protein isolates was pH dependent and was found to be higher at alkaline region of pH compared to acidic side. CPIB have poor foaming stability compared to CPIA at all pH range. The foam properties of cowpea flour have been shown to be of desirable characteristics for the production of several cowpea based food products.