
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2016) Volume 6, Issue 3
Results of this intervention will help to better understand the potential pathogenesis of T2DM. TCM compound preparations of SLP can not only provide a new chain of evidence of regulate intestinal flora of T2DM, but also compare the similarities and differences in transforming the structure of intestinal flora of T2DM patients with acarbose.
With the rapid economic development of china, people's living standards have been improved. However, accompanying chronic diseases have brought great impacts to people's health and the Quality of Life (QOL). According to the data released by the IDF, global diabetes population was 415 million in 2015, and it would rise to 642 million in 2040, with majority of type 2 diabetes [1]. It is known that type 2 diabetes is caused by interaction between genetic and environmental factors, and the pathogenesis of which is not yet completely clarified. In recent years, gut microbiota, the most important symbiotic microorganism system in body, has been confirmed closely related to metabolic diseases. With the further study, gut microbiota is expected to become the new targets for preventing and controlling type 2 diabetes.
More studies have suggested that the increase of diabetes should not be completely attributed to genetic background, changes in diet or physical activity decreases, the gut microbiota may play a more important role [2,3]. Gut is the first gate of absorption and utilization of nutrient. The gut microbiota can not only ferment and convert the indigested carbohydrates into short-chain fatty acids (SCFAs), but also regulate the genic expression and the energetic balance by affecting consumption and storage to participate in the energy metabolism of human body [4,5]. Furthermore, The disorder of gut microbiota structure could increase intestinal endotoxin level and intestinal permeability, promote LPS into blood, cause endotoxemia stimulate inflammatory factor secretion, arouse low grade inflammation, and result in obesity, type 2 diabetes or other metabolic disease finally [6]. Thus, gut microbiota may play an important role in the occurrence and development of obesity, insulin resistance (IR) and type 2 diabetes by participating in the energy metabolism and chronic inflammation. The intervention in intestinal flora may become one method for preventing and controlling diabetes. Studies had shown that probiotics and prebiotics could reduce the LPS level and inflammation, and improve glucose tolerance [7,8]. Transplanting the dung of thin male could improve insulin sensitivity and diversity of fecal bacteria in patients with metabolic syndrome [9]. It has been observed that Acarbose could regulate the gut microbiota, increase the intestinal bifidobacteria abundance, reduce the abundance of enterococcus in type 2 diabetes mellitus (T2DM) patients, meanwhile significantly reduce inflammatory factors and improve glucose and lipid metabolism [10]. Thus it could be seen that intervening in intestinal flora might contribute to the prevention and treatment of T2DM.
Traditional Chinese Medicine (TCM) has unique curative effect and long history in the preventing and controlling diabetes (Xiao Ke). The research, which is on TCM treating type 2 diabetes by affecting gut microbiota, is expected to be a new breakpoint clarifying the functional mechanism of Chinese medicine. Zhang et al. found the intestinal flora diversity in high-fat diet rats reduce under the action of berberine, 175 kinds of bacteria (containing harmful bacteria) declined or even disappeared, yet 93 kinds increased, among which Blautia and Allobaculum increased obviously, these two kinds of bacteria produce short-chain fatty acids. Meanwhile berberine significantly lowered endotoxin level and relieved inflammation [11]. A double-blind randomized, clinical trial (RCT) observed that medium or large dose of Ge Gen Qin Lian Tang reduced FBG, 2h-PGB level and improved IR significantly in T2DM patients. Based on analysis of bacterial type, Ge Gen Qin Lian Tang has also been found to inhibit opportunistic pathogens, such as Alistipes , fake butyrate vibrio, and meanwhile enrich some beneficial bacteria, such as Faecalibacterium which produces butyrate [12]. Besides, number of researches has shown that Chinese medicine functions well on regulating intestinal flora, especially the intestinal flora in human body [13-15]. Therefore, it might be a promising mechanism that TCM improves metabolism through regulating intestinal flora.
Sancai Lianmei Particle (SLP) is a TCM compound preparation. One previous multi-center RCT has indicated that SLP, had satisfying curative effect and security on newly diagnosed T2DM patients with good control of HbA1c and blood glucose in short-term (12 weeks) which was similar to metforminwithout any adverse events (AE). At the same time it improved pancreas islet function and reduced expression of inflammatory cytokines. An interesting phenomenon was observed during the experiment, SLP obviously lowered postprandial blood glucoseFPGbut no good effect on fasting glucose, (unpublished data). However the mechanism stays unclear.
Previous studies have shown that many TCM herbs, such as Panax Ginseng, Rhizoma Atractylodis Macrocephalae, Coptis Chinensis have the effect of regulating gut microbiota structure, Coptis Chinensis and Chinese herbal formula featuring Coptis Chinensis could improve glucolipid metabolism and gut microbiota structure significantly [11,12-14]. While Coptis Chinensis is the main herb in SLP, as the research result we mentioned above, it’s speculated that SLP improves glucolipid metabolism through regulating intestinal flora and reducing inflammatory state. Through the lateral comparison with metformin, the prophase research indicated that effect of SLP was surprisingly similar to acarbose [16] (both two kept satisfying control rate of HbA1c and blood glucose giving priority with reducing FPG, the effect was similar to metformin, and both of them). Moreoveracarbose was found well regulating inflammatory state of host and improving IR by regulating gut microbiota. Is it possible for such similar two kinds of medicines to share the same regulation mechanism of gut microbiota in T2DM patients? The question remains unclear and relevant research that compares the effective differences between two drugs on regulating structure characteristics of gut microbiota stays lacked. Therefore, we designed the project: the effect on gut microbiota structure of primarily diagnosed type 2 diabetes patients intervened by SLP and acarbose. Through the study, we look forwards to proving a potential function mechanism that SLP treats T2DM by regulating gut microbiota and further improving inflammatory state.
The primary objectives of the study are: (1) to assess the change of FPG, 2hPG, HbA1c level in 12 weeks, and (2) to examine the changes in the structure of gut microbiota in 12 weeks. The secondary objectives are: (1) to assess the change of weight, waistline and BMI in12 weeks; (2)the change of the oral glucose tolerance test (OGTT) and the Insulin release test in12 weeks; (3) the change of blood lipids and serum inflammatory factors in12 weeks; and(4) the change of HOMA-IR, ISI, and HOMA-β in12 weeks.
Study design
The present study is a randomized controlled trial. We have completed the registration on the Clinical Trial website. The number is ChiCTR-INR-16008440. The Ethics Committee of the Teaching Hospital of Chengdu University of Traditional Chinese Medicine (2015KJ-016) have approved the study design, study protocols and informed consent procedure.
All participants have to provide written informed consent. The patients were screened based on the inclusion criteria (HAb1c 7.5%-9.5%) before enter the group. Qualified patients who still met the inclusion criteria were involved in the study after controlling their diet and exercising moderately for 12 weeks.
Estimate the sample size and randomly divide qualified patients into experimental and control groups according with the 1:1 proportion. Besides, generate random numbers by SAS statistical software. Next treating patients with SLP in experimental group while acarbose in control group for 12 weeks. Above all, patients should keep their lifestyle and not use any other medicines which may influence the curative effect or the structure of the gut microbiota in the process. It is important to keep a record of various observed index and make a statistical analysis. The specific process of experiment is shown in Figure 1.
Participants and selection criteria
60 newly diagnosed type 2 diabetes outpatients would be recruited from the Endocrinology Department of the Teaching Hospital of Chengdu University of TCM.
Diagnostic criteria: According to the 1999 WHO diagnostic criteria for diabetes
The typical symptoms of diabetes (including polydipsia, polyuria and unexplainable weight loss), and in accordance with one of the following conditions.
Random blood glucose (venous plasma glucose) ≥ 11.1 mmol/L (200 mg/dl)
Fasting plasma glucose (fasting state refers to zero calorie intake for at least 8 hours) ≥ 7.0 mmol/L (126 mg/dl)
Oral glucose tolerance test (OGTT), 2 hours venous plasma glucose ≥ 11.1 mmol/L (200 mg/dl)
(2) The other day would be chosen to repeat measuring blood glucose if there is no diabetic symptoms.
Inclusive criteria mainly include:
At the age of 18-70
In accordance with the diagnostic criteria of T2DM
Body mass index from 18.5 kg/m2 to 30 kg/m2
No drug history or only a short-term (1 month) therapy for treating Type 2 diabetes, drug has been discontinued for 3 months before screening in the group
Poor blood sugar control after lifestyle intervention (HbA1c ranges 7.0%-9.0% and FBG ≤ 11.1 mmol/L
No antibiotics, micro ecological living bacteria preparation or lactulose used within 4 weeks before visiting doctor
The Voluntary to participate in the study, and sign the informed consent.
Exclusion criteria
Exclusion Criteria mainly include:
Type 1 diabetes, Adult occult autoimmune diabetes, malignant tumor, or other autoimmune disease
Gastrointestinal disease and (or) periodontal disease
Acute diabetes complications, including diabetic ketoacidosis, diabetes, high permeability of ketosis coma, hypoglycemia coma or severe unconsciousness hypoglycemia, etc
Severe gastrointestinal diseases, such as intestinal obstruction, intestinal ulcers or have obvious digestion, absorption, dysfunction
Heart failure, unstable angina, severe arrhythmia, myocardial infarction occurred in 12 months; SBP blood pressure >180 mmhg or DBP >100 mmhg
History of liver disease such as cirrhosis of the liver, hepatitis B or hepatitis C (excluding carriers), or the AST and ALT 2.5 times higher than normal ceiling
History of kidney disease or clinical diagnosis of renal insufficiency, serum creatinine is greater than 1.5 mg/dl (including 132.6 mmol/L)
With Other endocrine system diseases, such as hyperthyroidism and disease of grow in quantity of cortisol
Severe trauma, severe infections, surgery, or patients is not completely recovery after treatment
Mental illness, drug or other substance abuse or alcoholics
People who are accepted Steroids or is being treated for a malignant tumor
Allergies or allergic reactions to drugs used in this study
Pregnancy, nursing mothers, childbearing age women who take no effective contraceptive measures, or plan to pregnancy during test, positive results of urine HCG test
Participants take part in other drugs within three months
Family member’s disagreement
Generations
Demographic data: gender, age, nationality, occupation, education
History: a history of diabetes, family, medication, complications and treatment
Height (no shoes)
Weight (fasting, in the morning after excretion),
Waist, body mass index (BMI), waist to height ratio (WHtR); (detected at the 0, 4th, 8th, 12th week of drug intervention)
Therapeutic effect index
(1) The main outcome measures:
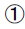 FPG, 2hPG: Glucose oxidase method.
FPG, 2hPG: Glucose oxidase method.
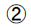 HbA1c: High performance liquid chromatography.
HbA1c: High performance liquid chromatography.
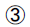 OGTT, Insulin release test: 75 g standard bun for oral administration, detect blood glucose and insulin levels at 0 min, 30 min, 60 min, 120 min, 180 min after feeding, no other food or drink with high heat or xanthine (such as caffeine) are allowed except for water. Glucose tolerance is tested by glucose oxidase method; Insulin releasing test: the chemiluminescence method.
OGTT, Insulin release test: 75 g standard bun for oral administration, detect blood glucose and insulin levels at 0 min, 30 min, 60 min, 120 min, 180 min after feeding, no other food or drink with high heat or xanthine (such as caffeine) are allowed except for water. Glucose tolerance is tested by glucose oxidase method; Insulin releasing test: the chemiluminescence method.
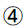 Blood lipid: total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C): fully automatic biochemical analyzer.
Blood lipid: total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C): fully automatic biochemical analyzer.
 Inflammation factors: c-reactive protein (CRP) and monocyte chemotactic protein 1 (MCP 1), tumor necrosis factor alpha (TNF alpha), serum lipopolysaccharide (LPS): enzyme-linked immunosorbent (ELISA)
Inflammation factors: c-reactive protein (CRP) and monocyte chemotactic protein 1 (MCP 1), tumor necrosis factor alpha (TNF alpha), serum lipopolysaccharide (LPS): enzyme-linked immunosorbent (ELISA)
Note:
1)  are detected at the 0, 4th, 8th, 12th week of drug intervention
are detected at the 0, 4th, 8th, 12th week of drug intervention
2)  ,
,  ,
,  are detected before the lifestyle intervention and at the 0, 12th week of drug intervention
are detected before the lifestyle intervention and at the 0, 12th week of drug intervention
3) The blood samples would be retained of item  according to the test requirements at the 0, 12th weeks of drug intervention; we use the unified detection kit and operate in strict accordance with the kit instructions
according to the test requirements at the 0, 12th weeks of drug intervention; we use the unified detection kit and operate in strict accordance with the kit instructions
(2) The secondary efficacy measures were calculated based on the data obtained from the 0 week and 12 weeks of insulin release as follows:
 HOMA-IR=(FPG × FINS)/22.5
HOMA-IR=(FPG × FINS)/22.5  ISIISI=1/(FPG × FINS)
ISIISI=1/(FPG × FINS) 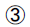 HOMA-β: HOMA-β=(20 × FINS)/ (FPG-3.5)
HOMA-β: HOMA-β=(20 × FINS)/ (FPG-3.5)
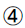 AUCI/AUCG/AUC=FPG/2+1 h numerical value +2 h numerical value +3 h numerical value /2. The data above are obtained from insulin release test.
AUCI/AUCG/AUC=FPG/2+1 h numerical value +2 h numerical value +3 h numerical value /2. The data above are obtained from insulin release test.
Structural modulation of gut microbiota
Sample collection and DNA extraction: collect fresh stool samples in the aseptic boxes at 0 and 12th weeks of drug intervention. Take the sample into the refrigerator of -70oC immediately. Send the samples to the central laboratory for extracting the DNA by QIA amp DNA Stool Mini KitQiagen) and inspecting the quality by Qubit2.0.
DNA amplification and DNA sequencing: Amplify the 16S rRNA genes’ V3 regions of fecal DNA by PCR, and sequence the 16s rDNA by Ion Torrent PGMafter the amplification results being qualified. The sequencing reads of target genes is single-ended with 200nt reads. All processes of DNA PCR amplification, Library Construction, Library quality inspection and DNA sequencing were designed by assigned company.
Note: Shanghai Biotechnology Corporation assist in completing the DNA extraction, quality inspection, amplification, sequencing, and follow-up data processing of gut microbiotaAbove.
Intervention measures
Test group: Sancai Lianmei Particle (SLP), each bag contains 10 g.
Drug component: Ginseng, Asparagus Cochinchinensis, Radix Rehmanniae, Rhizoma Coptidis and Cortex Cinnamomi
Directions and dosage: Three times a day, drink one bag with warm water before each meal.
Control group: Acarbose Tablets, Bayer HealthCare Company, each tablet contains 50 mg. Directions and dosage: Take two tablets three times daily with meals, chewing with the first bite of staple food.
Basic treatment (For experimental group and the control group): According to "Chinese Diabetes Prevention Guide" (2013 Edition), diabetes education, individualized diabetes diet control and diabetes exercise guidance program would be given to patients.
Sample size
Each group contains 30 patients. Allowing for the addition of 20% shedding rates, 72 patients would be counted in this small-scale tentative study totally.
Statistical analysis
SPSS for Windows, version 21.0 (Chicago, USA) will be used for the statistical analyses. Values will be expressed as mean ± SD. Student’s ttest will be utilized to compare indexes between the study groups, Non-normal distribution data using nonparametric test. HOMA relevant indicators for the statistical analysis after taking the natural logarithm. Pearson correlation analysis and partial least squares (PLS) regression analysis will be used to analyze correlation between the intestinal flora with inflammatory factor, blood sugar, blood fat, body mass index (BMI) ,and so on. The significance level will be set at 95% (α=0.05). Intestinal flora using bioinformatics and multivariate statistical analysis (Figure 2).
Bioinformatics and multivariate statistics
High-quality sequence alignments were performed using Ion Torrent PGM. Sequence clustering by CD-hit and OUT delineation by MOTHUR was performed as described (http://www.mothur.org/). The representative sequences of operational taxonomy units (OTUs) with their relative abundance were used to calculate Rarefaction analysis and Shannon diversity index by MOTHUR. The classification of each samples species identification by MOTHUR (version: 1.31.2).
The phylogenetic tree and the relative abundance table of representative sequences of OTUs were used for UniFrac principal coordinate analysis (PCoA). Redundancy analysis was performed using Canoco5 according to the manufacturer’s instructions. The specific analysis process of intestinal flora is shown in Figure 2.
Results of this intervention will help to better understand the potential pathogenesis of T2DM.T CM compound preparations of SLP can not only provide a new chain of evidence of regulate intestinal flora of T2DM, but also compare the similarities and differences in transforming the structure of intestinal flora of T2DM patients with acarbose.
The authors have declared that no competing interest exists.