Research Article - (2024)Volume 8, Issue 1
In recent years, the Cerium Oxide Nanoparticles (CeO2 NPs) will be used more and more widely, and their effects were also be concerns about on human health, particularly on the reproductive system. The previous studies have indicated that the testicles and sex hormones in males were be damaged by CeO2 NPs with long-term exposure. The effects of CeO2 NPs with different physical and chemical parameters, including size, shape, and surface coating about on male reproductive toxicity are explored in this study. This study analyzes the toxic effects of CeO2 NPs on male reproduction from the aspects of germ cells, sperm structure, blood-testis barrier, pituitary gonadotropins, and epididymis. Those findings will provide a theoretical basis and scientific evidence for the use of CeO2 NPs in the future.
Nano-cerium oxide; Male-reproductive system; Toxic mechanism
BBB: Blood-Brain Barrier; Ce: Cerium; CeO2 NPs: Cerium Oxide Nanoparticles; FSH: Follicle-Stimulating Hormone; GnRH: Gonadotropin-Releasing Hormone; HPG: Hypothalamic Pituitary Gonadal axis; HO2•: Hydrogen peroxide radicals; LH: Luteinizing Hormone; LPO: Lipid Peroxidation; LOOH: Lipid Hydroperoxide; LO•: Lipid Peroxyl radical; MDA: Malondialdehyde; O2-: Oxygen ion; OH•: Hydrogen peroxide radicals Hydroxyl radicals; PAA: Polyacrylic Acid; PRL: Prolactin; RNS: Reactive Nitrogen Species; ROS: Reactive Oxygen Species
Cerium oxide nanoparticles (CeO2 NPs) have a wide range of applications in drug delivery, bio-imaging, and other fields [1,2]. However, the studies have shown that CeO2 NPs may pose a potential risk to human health, particularly to the male reproductive system [3,4]. Therefore, this study focuses on the effects of CeO2 NPs about male fertility, including sperm structure, blood-testis barrier, and testicular function, and summaries the mechanisms of CeO2 NPs on male reproductive toxicity simply. These research results provide an important scientific reference for the safety of CeO2 NPs.
The effects of cerium oxide nanoparticles on the male reproductive system in physicochemical parameter
Cerium (Ce) is one of the most abundant rare-earth metals in the Earth's crust, accounting for about 0.0046% by weight, and it belongs to the lanthanide group of elements in the periodic table [5]. Unlike most rare earth metals, cerium exists in two states (Ce3+ and Ce4+), that the oxidation state of Ce4+ is usually considered to be more stable than Ce3+, and the electronic structure of Ce4+, [Xe] 4f0, is more stable than that of [Xe] 4f1 of Ce3+ [6]. A mixture of both 3+ and 4+ states will exist on the surface of CeO2 NPs [7]. Compared to conventional organic antioxidants, CeO2 NPs are multi-enzyme activity because of Ce4+/Ce3+ redox cycle. This enzymatic activity scavenges free radicals, provides protection from ionizing radiation, and attenuates oxidative stress [8].
Particle size: CeO2 NPs with the smaller sizes are considered to be more toxic, because of Ce3+/Ce4+ ratios with higher surface [9]. Comparing nanoscale (particle sizes of ~40 and 5-10 nm) and a microscale (particle sizes <5000 nm) CeO2 NPs material in rats under a 28-day inhalation toxicity, the study showed that CeO2 NPs with the size of 40 nm caused the greatest damage in the exposure levels, while CeO2 NPs (<5000 nm) induced the greatest degree of lung inflammation and damage [10]. In addition, a study using CeO2 NP particles of 30 nm in size prepared by supercritical synthesis investigate acute oral toxicity and tissue distribution using a single administration. The results of the study showed that the cumulative mean values of CeO2 NPs were increase in various tissues including the testis [11]. Another study explored the effect of CeO2 NPs (3-5 nm) on tissue development and apoptotic gene expression in the fetal testis Nuclear Magnetic Resonance Imaging (NMRI) of 6-day-old allopatric mice. This study found that mRNA expression of Bax, cysteinyl asparaginase-2, and Gsk2-β genes was significantly decreased in testicular tissues of the experimental group, compared to the control group, and demonstrated that the injection of CeO2 NPs affects the development of neonatal testicular tissue [12]. Préaubert et al. synthesized CeO2 NPs with a particle size of 7 nm in ellipsoidal microcrystalline under acidic conditions and analyzed the genotoxicity of CeO2 NPs by comet assay. The transmission electron microscopy was used to observe the content of CeO2 NPs on the plasma membrane of exposed human spermatozoa, and the study found that CeO2 NPs under very low concentrations can cause significant DNA damage to human spermatozoa. The genotoxicity was inversely related to the concentration of CeO2 NPs [4].
Shape: The toxicity of CeO2 NPs is also related to shape. Depending on the shape and surface charge of the nanoparticles, the degree of migration of the nanoparticles can be accelerated to 60 orders of magnitude [13]. Forest et al. reported that rod-shaped CeO2 NPs increased the toxicity of RAW264.7 macrophages, excluding cubic and octahedral CeO2 NPs [14]. Cotena et al. observed that cuboctahedral and rod-shaped CeO2 NPs had cytotoxic effects on human hepatocellular carcinoma cells, and it was found that cubic and rod-shaped CeO2 NPs exhibited the highest and lowest toxicity, respectively [15]. In addition, Gatoo et al. found that the fertilization rates of CeO2 NPs (0.01 and 100 mg/l, ellipsoidal, ~7 nm) were significantly lower than those at very low concentrations (0.01 mg/l). Meanwhile, the damage significantly was found in the spermatozoa and oocytes of DNA, which may be a result of the genotoxic effects of CeO2 NPs on gametes, disruption of gamete-gamete interactions, and oxidative stress induced by CeO2 NPs [16].
Surface coating: The coating of CeO2 NPs plays an important role in their toxicity. The coating agents typically cover the surface of the nanoparticles, that are very stable by inhibiting aggregation. In one study, Polyacrylic Acid (PAA) was used to stabilize CeO2 nanoparticles, and their toxicity was compared to the uncoated form. The effect of the coating resulted in a significant increase in toxicity [17]. Another study found that CeO2 NPs with the coating of amorphous silica reduced the inflammatory response in the lungs [18]. The citrate ions were coated on CeO2 NPs and deposited as precipitates, resulting in enhanced interaction with cells. Thus, the citrate-coated nanoparticles showed toxicity and moderate genotoxicity at high concentrations, whereas PAA-coated nanoparticles were stable and did not show toxicity [19]. Zinc Zn-CeO2 NP particles synthesized by green sol-gel method were proved to be non-toxic by in vitro experiments on mouse neuroblastoma cell line (NeurO2A) [20]. In another study using the biopolymer carrageenan hydrogel as a capping agent for CeO2 NP particles showed that the obtained CeO2 NP particles had no toxic effects on NeurO2A cells after acute administration [21].
The effects of cerium oxide nanoparticles on male reproductive system
The effect of cerium oxide nanoparticles on testes: Some studies suggest that exposure to CeO2 nanoparticles might have adverse effects on testicular function. These effects could include:
The effects of cerium oxide nanoparticles on spermatogenic cells and spermatozoa structure: Spermatogonia are key cell types in the reproductive system, undergoing a series of differentiation and developmental processes that culminate in the formation of spermatozoa [22]. The effects of CeO2 NPs on spermatogenic cells were found to be mainly in terms of cell cycle, meiosis, number, and activity [23,24]. Qin et al. found that oral administration of CeO2 NPs (32 days) to male mice resulted in degenerative changes in testicular tissues of experimental CeO2 NPs (20 and 40 mg•kg-1) mice compared to the controls, such as atrophy or necrosis of spermatogonial tubules, loosening of spermatogonial epithelial cell adhesion or detachment, spermatogenesis, spermatozoa loss, and apoptosis of mesenchymal tissues. The histological studies also showed that a variety of cells, such as Leydig cells, supporting cells, spermatogonia, primary spermatocytes, and spermatids, were significantly reduced [25]. In addition, CeO2 NPs may interfere with the meiotic process of spermatogonia, leading to chromosomal abnormalities, thus affecting the quality of spermatozoa [23]. Preaubert et al. showed that the mouse exposure to CeO2 NPs, that resulted in a significant increase damage on DNA [26]. Lee et al. showed that the mice exposed to different concentrations of CeO2 NPs for 5 days, and it led to downregulation of the expression levels of relevant genes, which negatively impacted pre-pubertal spermatogenesis and maintenance of germ cells [27]. Préaubert et al. found that human spermatozoa exposed to low concentrations of CeO2 NPs induced DNA damage significantly by in vitro experiments and the damage was inversely proportional to the concentration of CeO2 NPs [4]. Hosseinalipour et al. found that the male mice exposed to continuous administration of CeO2 NPs (50 and 100 mg•kg-1) for 35 days, the seminiferous tubule diameter, epithelial height of seminiferous tubules, and spermatogenesis index decreased in the testes, along with a significant reduction in sperm parameters (counts, viability, vitality, and morphology) [28]. The results of these studies indicate that CeO2 NPs significantly affect the cell cycle, meiosis, and other processes in spermatogenic cells, which in turn affect sperm count, average motility, and shape. In addition, exposed to CeO2 NPs may lead to the damage of sperm DNA and sperm quality. The testicular tissue degenerative changes under CeO2 NPs, and the production of sperm reduced that have a significant effect on the health of human reproductive.
The effects of cerium oxide nanoparticles on the blood-testis barrier: The blood-testis barrier is a physical barrier between the lumen of the testicular capillaries and the spermatogenic tubules, which plays an important role in maintaining the morphology and function of spermatozoa, controlling the permeation and filtration of blood-testis fluids and exogenous substances, and sustaining the immune isolation of spermatozoa [29]. Artimani et al. found that CeO2 NPs may inhibit the function of mouse testicular mesenchymal stromal cell tumor (Leydig) cells and reduce testosterone production, which in turn affects the function of testicular supporting cells (Sertoli) and the stability of the blood-testis barrier [30]. Adebayo et al. injected mice intraperitoneally with different concentrations of CeO2 NPs with saline and found that the level of testosterone produced by Follicle-Stimulating Hormone in response to testosterone-secreting Leydig cells reduced significantly under CeO2 NPs, thereby inhibiting the secretion of testosterone [31]. Nemati et al. showed that the number of spermatogonia and sertoli cells in the testes of 2-day-old neonates significantly reduced under CeO2 NPs intraperitoneally to mice of different gestational days [24].
Hamzeh et al. intraperitoneally injected CeO2 NPs (5 mg•kg-1) given to mice for 7 days consecutively and found that CeO2 NPs significantly induced oxidative stress in the testis, resulting in a significant reduction in sperm counts, motility, sperm viability, and testosterone levels [32]. Testosterone is an important protein that regulates the integrity of the blood-testis barrier and an essential molecule for the maintenance of sertoli cell junctions [33]. In summary, CeO2 NPs may have an impact on the integrity of the blood-testis barrier and the function of cells within the testis as shown in Table 1. These findings suggest that CeO2 NPs have a potential impact on maintaining the stability of the bloodtestis barrier and normal spermatogenesis.
| Experimental subject | Nano particle |
Shape | Particle size | Concentration | Conclusion | References |
|---|---|---|---|---|---|---|
| Human sperm | CeO2 NPS | Oval shape | 7 nm | 0.01、0.1、1-10 mg·L-1 | CeO2 NPs are genotoxic to human cell lines, and very low concentrations of CeO2 nanoparticles can induce significant DNA damage in human spermatozoa | [4] |
| Pregnant NMRI mice | CeO2 NPS | - | <5 nm | 10、25、80、250 mg/kg.bw | Administration of CeO2 during pregnancy may affect neonatal testicular tissue and blood biochemical indexes in a dose-dependent manner | [24] |
| Adult male C57BL/6J mice | CeO2 NPS | Cubic crystal | 27.62 ± 3.01 nm | 10、20、40 mg/kg.bw | CeO2 NPs at 20 mg/kg and 40 mg/kg increased elemental Ce content in testes, testicular histopathological patterns and sperm DNA damage, and decreased testicular mass, DSP and sperm motility. The levels of testosterone and the activities of marker enzymes were significantly decreased, the mRNA expression levels of steroidogenic genes such as Star, P450scc, P450c17, 3β-Hsd, and 17β-Hsd were down-regulated, and the mrna and protein expression levels of SF-1 were changed. | [25] |
| B6-CBA-F1 mice | CeO2 NPS | Ellipsoid shape | 7 nm | 0.01、100 mg·L-1 | Very low concentration (0.01 mg·L-1) significantly reduced the fertilization rate and DNA damage in vitro fertilization. The 100 mg·L-1CeO2 NPS accumulated along the plasma membrane of sperm and the zona pellucidum of oocytes. | [26] |
| Mouse testis fragments | CeO2 NPS | - | <25 nm | 10、30、50 μg/mL | Significantly reducing the number of undifferentiated and differentiated germ cells, 50 μg/mL CeO2 NP reduced Sox9 protein expression and steroidogenic enzyme mRNA expression levels in mouse testicular fragments. | [27] |
| Adult balb/c mice | CeO2 NPS | - | - | 5 mg/kg of NPs for 7 days | The levels of MDA, ROS and PC were increased、the GSH level was decreased, and the testis was severely damaged. Sperm number, motility, sperm motility, and testosterone levels were significantly decreased, and the number of abnormal sperm was significantly increased. | [32] |
| Adult male NMRI mice | CeO2 NPS | - | 30 nm | 50、100 mg/kg.bw of NPs for 35 days | The diameter, epithelial height and spermatogenesis index of sperm tubules in the CeO2 NPs group were significantly decreased, while the proportion of immature sperm and sperm with DNA damage was significantly increased. | [28] |
| Adult balb/c mice | CeO2 NPS | - | <10 nm | 100、200、300 μg/kg | CeO2 NPs significantly decreased the levels of hemoglobin and red blood cells. 100 μg/kg CeO2 NPs reduced testosterone levels by 23% the levels of MDA in the testis of mice treated with 100, 200 and 300 μg/kg CeO2 NPs increased by 103%, 106% and 135%, respectively. | [31] |
| Human NB cellline (IMR32) | CeO2 NPS | - | <25 nm | 10-200 mg/mL 24 h incubation | CeO2-NPs induce oxidative stress and genotoxicity at concentration above 100 mg/mL | [34] |
| Neuro2A cells | CeO2 NPS | Fluorescent stone cubic structure | <10 nm | 0-175 μg/mL | Dose-dependent toxicity with effective concentration 10 μg/mL | [35] |
| Neuro2A cells | CeO2 NPS | Fluorescent stone cubic structure | <50 nm | 0-100 μg/mL | The metabolic activity was decreased in a concentration dependent manner at concentration above 25 μg/mL | [36] |
| Neuro2A cells | CeO2 NPS | Fluorescent stone cubic structure | 20-40 nm | 0-125 μg/mL | Dose-dependent toxicity with effective concentration 30 μg/mL | [37] |
Table 1: The effects of cerium oxide nanoparticles on male reproductive system.
The effects of cerium oxide nanoparticles on Hypothalamic Pituitary Gonadal axis (HPG): It has been shown that nano- and micro-sized CeO2 can cross the Blood-Brain Barrier (BBB) and accumulate in the brain [38]. The accumulation of particles in the brain may be detrimental to hormone production. The menstrual hormones including Gonadotropin-Releasing Hormone (GnRH), Follicle-Stimulating Hormone (FSH), and Luteinizing Hormone (LH) are naturally produced by the hypothalamus and pituitary in the Hypothalamic-Pituitary-Gonadal HPG axis, and play an important role in spermatogenesis [23,39]. Prolactin (PRL) is a polypeptide hormone secreted mainly by lactation cells of the pituitary gland, which controls the production of LH and FSH by regulating Gonadotropin-Releasing Hormone (GnRH) via the hypothalamus (a feedback mechanism) [40]. Adebayo et al. found that FSH, LH, and prolactin by 25%, 26%, and 13%, respectively under CeO2 NPs (200 μg/kg) [31]. Thus, the accumulation of CeO2 NPs in tissues, such as the brain, can indirectly interfere with reproductive development by disrupting the balance of HPG axis hormones [41].
In addition, CeO2 NPs may directly affect the function of testicular. The testis is the male gonad that produces sperm and is a key component of the HPG axis [42]. The study investigating the effects of arsenic oxide particles on several organs (lungs, liver, kidneys, spleen, brain, testes, and epididymis) showed that cerium could be detected in all investigated cha organs after single and repeated exposures [38]. Nemati et al. found that the high doses of CeO2 NPs can have destructive effects on fetal renal development in neonatal mice, it affects adrenal hormones and reduces testosterone synthesis [43]. In addition, the presence of CeO2 NPs may not only interfere with the normal function of testicular cells, but also affect the count and viability of sperm, the levels of testosterone hormone, and HPG [44]. Overall, CeO2 NPs may have indirect effects on neurohormonal homeostasis and directly interfere with testicular function. These results suggest that CeO2 NPs may adversely affect reproductive development as shown in Table 1.
The effects of cerium oxide nanoparticles on epididymis: The epididymis provides an important microenvironment for sperm maturation [45]. CeO2 NPs lead to oxidative stress and destory the structure of mitochondrial, dysfunction in energy metabolism and adversely affect the quantity and quality of epididymal spermatozoa [46]. CeO2 NPs were detected in vivo in the liver, spleen, brain, testis, and epididymis of rats after 6 h of exposure [47]. Hosseinalipour et al. found that there were testicular tissue alterationsin the mice by oral infection of CeO2 NPs (50 and 100 mg•kg-1 for 35 days), that may reduce the quality of sperm parameters. The tubular diameter, epithelial height of SNT and spermatogenesis index were significantly reduced in the experimental group in vitro embryo development, and the immature spermatozoa and its DNA damage was significantly increased in the groups treated with CeO2 NPs as compared to the control group. These results suggest that CeO2 NPs can increase chromatin abnormalities in spermatozoa and significantly reduces the percentage of viable spermatozoa [28].
The mechanisms of male reproductive toxicity of cerium oxide nanoparticles
There is still no report on the mechanism of CeO2 NPs with the male reproductive system. This study suggest that the toxicity of CeO2 NPs may be from the oxidative stress. CeO2 NPs with highly active intrinsic defects (oxygen vacancies) can store and release oxygen with autocatalytic properties [48]. The two electrons from the oxygen atom are transferred to two Ce4+ ions in the vicinity of the vacancies, that reduced in Ce3+, and then it leads to the release of hydroxyl radicals (OH-) and induces the development of oxidative stress as shown in Figure 1. Oxidative stress causes damage to lipids, proteins, and DNA, and ultimately leads to cell death, DNA damage, and lipid peroxidation, among others from the levels of antioxidants and Reactive Oxygen Species (ROS) [49]. The studies reported that nanoparticles can induce oxidative stress in the testis department, which may be related to the biological environment of CeO2 NPs [15]. Oxidative stress come from the overproduction of ROS, Nitrogen-Reactive Substances (NRS), or DNA-reactive aldehydes [50]. CeO2 NPs produce large amounts of ROS and RNS and catalyze Fenton-like reactions by redox cycling with H2O2 to produce oxygen radicals [51]. In addition, nanoparticles can induce Lipid Peroxidation (LPO) in the studies [52]. Bartsch et al. have demonstrated that there is an increase in the production of malondialdehyde (LPO), after exposure to CeO2 NPs (48 h) nano concentrations of CeO2, that damage to DNA and proteins [50]. Auffan et al. have shown that RNS, ROS, and LPO from CeO2 NPs under long-term exposure conditions,can induce DNA damage during DNA replication [53].
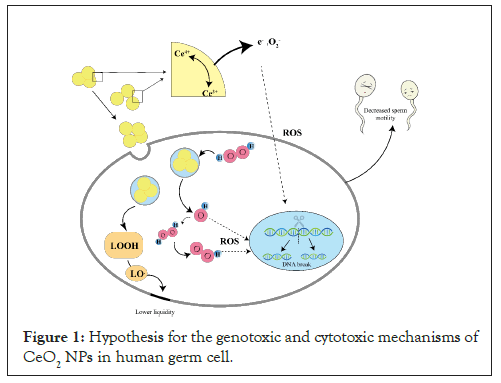
Figure 1: Hypothesis for the genotoxic and cytotoxic mechanisms of CeO2 NPs in human germ cell.
CeO2 NPs consist of Ce3+ and Ce4+ on their surface, existing in a mixed valence state. Valence transitions in CeO2 NPs generate electrons (e-) and oxygen ion (O2-), which can lead to the production of Reactive Oxygen Species (ROS). Hydroxyl radicals (OH•) are formed within the cell from the reaction of hydrogen peroxide (H2O2) with Ce3+, while hydrogen peroxides (HO2•) are generated from the reaction of H2O2 with OH•. These ROS induce oxidative stress in the cell. The presence of oxidative stress can result in DNA damage, affecting the integrity and stability of the genetic material. This can lead to reduced sperm viability and decreased sperm numbers. Furthermore, Ce3+ can break down lipid peroxides (LOOH) into lipid peroxyl radicals (LO•), which can initiate lipid peroxidation. Lipid peroxidation can impair membrane integrity, leading to reduced membrane fluidity.
CeO2 will be focused on male reproductive toxicity in the future, because of negative impacts, such as sperm, testicular function, and fertility. The studies have shown that the toxic of CeO2 NPs are closely related to their physicochemical properties. CeO2NPs can induce the reactive from oxygen radicals (ROS) and the changes in Malondialdehyde (MDA) concentration, which is a major cause of oxidative stress. Although the deleterious effects of CeO2 NPs were confirmed from the clinical studies, it is not yet possible to clarify the mechanisms from CeO2 NPs on the male reproductive system. This study will provide a deeper understanding for safety use of CeO2 NPs.
Writing-original draft preparation: WB Y, ZH J and Y Y; writing-review and editing: all the authors; supervision: X Z; project administration: FJ Q; funding acquisition: FJ Q and X Z. All the authors read and agreed to the published version of the manuscript.
The authors acknowledge the financial support by the National Natural Science Foundation of China (32302185).
This article has no additional data.
The authors declare that there are no conflicts of interest.
Citation: Yu W, Jia Z, Yang Y, Zheng W, Wang Z, Dong X, et al (2024) The Effects of Nano-Cerium Oxide on Male-Reproductive System. J Pharma Reports. 08:200.
Received: 11-Dec-2023, Manuscript No. JPR-23-28424; Editor assigned: 14-Dec-2023, Pre QC No. JPR-23-28424 (PQ); Reviewed: 29-Dec-2023, QC No. JPR-23-28424; Revised: 05-Jan-2024, Manuscript No. JPR-23-28424 (R); Published: 12-Jan-2024 , DOI: 10.35248/JPR.24.8.200
Copyright: © 2024 Yu W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.