Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Review Article - (2021)Volume 12, Issue 2
Objectives: Despite the potential health risks, Paracetamol is commonly misused for management of hangover among regular users of alcohol. Studies on the combined use of the two drugs are limited and controversial. The aim of this study was to evaluate the effects of the interaction of the two drugs on the biological parameters of a rat model.
Methods: Animals were divided into twelve groups. The negative and positive controls received distilled water and alcohol, respectively. Alcohol was administered at 2.5, 3.5 and 4.5 g/kg orally for 4 weeks. Paracetamol was given at doses of 40 and 400 mg/kg. Half of the other groups received combined doses of the two drugs. Hematological and blood chemistry were determined using auto-analyzers while histostructure was scored under light microscopy.
Results: Alcohol induced a dose and time dependent gain on body weight, but when used in combination with paracetamol the effect was mixed. When used independently and combined, the drugs did not affect (p>0.05) the hematological profiles. For blood chemistry, the drugs caused a dose dependent elevation of liver enzymes, bilirubin, urea, reduced albumin levels and various degrees of liver and renal pathology.
Conclusion: In moderate doses, Paracetamol is safe but high doses and chronic use of alcohol causes hepatotoxicity. Individually, alcohol and Paracetamol have a low risk of renal damage but when used together the risk is increased. Thus, regular use of Paracetamol in management of alcohol induced hangover is discouraged as this increases the risk of liver and kidney diseases.
Paracetamol; Alcohol; Hepatoxicity; Nephrotoxicity
Alcohol is one of the most popular recreational drug that is legally available. It is often consumed to produce pleasurable effects such as inhibition, euphoria, sedation and mild anesthesia [1,2]. Moderate consumption of alcohol has beneficial effects such as lowered risk of developing diabetes, liver and heart diseases [3-5]. However, excessive usage has serious negative health, social and economic consequences [6,7].
The most important clinical manifestation of chronic alcohol abuse is Alcoholic Liver Disease (ALD). The condition encompasses a range of disorders involving alcoholic fatty liver (steatosis), hepatitis and cirrhosis [8]. Alcoholic fatty liver occurs as a result of increased NADH/NAD+ ratio which promote free fatty acids and triglyceride synthesis while inhibiting beta-oxidation of free fatty acids [9]. Alcohol also causes induction of cytochrome P450 and subsequent production of reactive oxygen species [10]. The reactive oxygen species reduce antioxidant glutathione and stimulates hepatic stellate cells that are responsible for the fibrosis and hence cirrhosis [11]. Other mediators of alcoholic hepatitis and cirrhosis include acetaldehyde adducts, inflammatory cytokines and endotoxins [12].
Chronic abuse of alcohol has also been implicated in kidney damage [13]. In rats, the drug can produce significant renal dysfunction and abnormalities in morphological structure [14,15]. The kidney is susceptible to alcohol-induced oxidative damage because it has high content of long-chain polyunsaturated fatty acids which promote lipid peroxidation [16,17].
Paracetamol is a Non-Steroidal Anti-Inflammatory Drug (NSAID) that is widely used to treat pain and fever. At therapeutic doses, paracetamol is well tolerated and has lower incidences of adverse effects compared to other NSAID such as aspirin [18]. Due to its safety paracetamol is widely misused for the relief of alcohol induced hangover symptoms.
Studies on the combined effects of alcohol and paracetamol are limited and controversial. One report suggests that paracetamol at therapeutic doses is safe and effective even in chronic alcoholics [19]. However, in another study the drug can cause liver and kidney diseases in patients who are glutathione depleted or who take drugs such as alcohol that stimulate the cytochrome P450 enzymes [20].
The major weakness of these studies is that they are not controlled. There is, therefore, need to conduct a controlled study that examines the effects of paracetamol and alcohol interaction on the physical and biological parameters of a rat model.
Laboratory animals
Adult Winster albino rats of 3-4 weeks age were used in this study. The experiments were carried out in accordance with the 1996 guidelines for the care and use of laboratory animals [21].
Chemicals
Alcohol (Smirnoff Vodka®, 37.5%) was a product of East Africa Breweries Limited (Nairobi, Kenya). Cipladon®1000 (Cipla Ltd., Mumbai, India) containing 1000mg paracetamol was sourced from Rangechem Pharmaceuticals (Nairobi, Kenya).
Experimental design
The rats were separated into twelve groups of five animals each. The negative control group was treated with distilled water. While the rest were given either alcohol or paracetamol or a combination of both. The treatments were done orally for a total of four weeks. Details of the treatment regimens are presented in Table 1.
| Group | Treatment |
|---|---|
| A | Distilled water (negative control) |
| B | 2.5 g/kg alcohol |
| C | 3.5 g/kg alcohol |
| D | 4.5 g/kg alcohol |
| E | 40 mg/kg paracetamol |
| F | 400 mg/kg paracetamol |
| G | 2.5 g/kg alcohol+40 mg/kg paracetamol |
| H | 2.5 g/kg alcohol+400 mg/kg paracetamol |
| I | 3.5 g/kg alcohol+40 mg/kg paracetamol |
| J | 3.5 g/kg alcohol+400 mg/kg paracetamol |
| K | 4.5 g/kg alcohol+40 mg/kg paracetamol |
| L | 4.5g/kg alcohol+400mg/kg paracetamol |
Table 1: Experimental design.
Physical parameters of study animals
Individual body weights of rats were determined shortly before the start of the experiment and weekly thereafter until the end of the study. The general physical appearance and behavior of rats was monitored throughout the experiment period.
Blood collection and analysis
At the end of the experiment blood was collected for hematological, blood sugar and biochemical analysis as described below.
Hematological analysis
EDTA blood was collected from the tail and analyzed using Mindray BC-5300 auto hematological analyzer (Shenzen Mindray Bio-Medical Electronics Co., Ltd, Shenzhen, China) as per the manufacturer’s recommendations [22].
Blood glucose levels
Tail was placed at one end of the glucometer strip and reading taken after 10 seconds using a glucometer (On Call Plus®, ACON laboratories, San Diego, USA).
Biochemical analysis
For biochemical analysis, rats were euthanized using diethyl ether and blood drawn using sterile needle by cardiac puncture. Serum was processed and biochemical analyses were performed using a chemistry analyzer (Selectra ProS®, ELITech group clinical systems, Milsbeek, Netherlands).
Histopathological analysis
Liver and kidney tissues were removed and weighed. The tissues were fixed in 10% formalin, embedded in liquid paraffin, sectioned, stained with hematoxylin and eosin, and examined under light microscopy [23,24].
Data analysis
Comparisons of weights, biochemical and hematological parameters were done using one-way Analysis of Variance (ANOVA). For multiple comparisons Tukey’s post hoc test was applied.
Effects of alcohol and paracetamol on morphological characteristics of rats
Control group rats and those treated with 2.5 g/kg alcohol, 40 mg/kg of paracetamol and 2.5 g/kg of alcohol plus 40 mg/kg of paracetamol were active, had glossy coat and were of good appetite throughout the treatment period. Those that received 3.5 g/kg or 4.5 g/kg of alcohol were initially active and had good appetite. However, from days sixteen and nine respectively, they had poor appetite. The remaining five groups of rats, which were either treated with 400 mg/kg of paracetamol or co-treated with alcohol and paracetamol, had rough hair coat, poor appetite, were inactive and they huddled in a corner of the cage.
Effects of alcohol and paracetamol on body weight of rats
The control group recorded the highest weight gain of 31.8 g in the first week but it also displayed the lowest weight gain of only 7 g in the fourth week. Multiple comparisons of the mean weight change of the control group and other treatment groups showed that in the first week of study, rats that were treated with either alcohol or paracetamol did not have a significant (p>0.05) change on body weight. However, those that received both drugs at doses of 2.5 g/kg alcohol plus 40 mg/kg of paracetamol, 3.5 g/kg of alcohol plus 40 mg/kg paracetamol, 3.5 g/kg of alcohol plus 400 mg/kg paracetamol and 4.5 g/kg of alcohol plus 40 mg/kg paracetamol had significantly (p<0.05) low weight gain. In the second and third week, there were insignificant change in weight in all groups except for the group that was treated with 4.5 g/kg of alcohol plus 400 mg/kg of paracetamol that recorded a significant (p<0.05) weight gain in the latter.
In week four, the groups treated with 3.5 g/kg or 4.5 g/kg of alcohol recorded significant (p<0.05) weight gain. A similar finding was observed in rats treated with 40 mg/kg paracetamol. There was also significant weight increase in all the groups that were given the two drugs together except for the one that received the highest doses of alcohol plus paracetamol (p>0.05) (Figure 1).
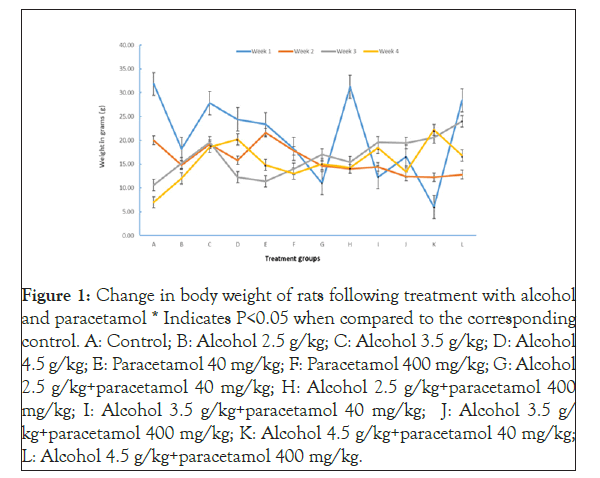
Figure 1: Change in body weight of rats following treatment with alcohol and paracetamol * Indicates P<0.05 when compared to the corresponding control. A: Control; B: Alcohol 2.5 g/kg; C: Alcohol 3.5 g/kg; D: Alcohol 4.5 g/kg; E: Paracetamol 40 mg/kg; F: Paracetamol 400 mg/kg; G: Alcohol 2.5 g/kg+paracetamol 40 mg/kg; H: Alcohol 2.5 g/kg+paracetamol 400 mg/kg; I: Alcohol 3.5 g/kg+paracetamol 40 mg/kg; J: Alcohol 3.5 g/ kg+paracetamol 400 mg/kg; K: Alcohol 4.5 g/kg+paracetamol 40 mg/kg; L: Alcohol 4.5 g/kg+paracetamol 400 mg/kg.
Effect of alcohol and paracetamol on hematological parameters of rats
Treatment with alcohol or paracetamol, either individually or combined, did not have a significant (p>0.05) effect on the hematological profile of the study animals. The parameters studied were total white blood cells, lymphocytes, monocytes and granulocytes counts, red blood cells counts, mean corpuscular volume, hematocrit, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, hemoglobin, platelet counts, mean platelet volume, plateletocrit and platelet distribution width.
Effects of alcohol and paracetamol on biochemical parameters
Functions of liver: Most of the treatments with alcohol or paracetamol were found not to affect the serum albumin levels. However, rats given 4.5 g/kg of alcohol, 400 mg/kg paracetamol, and 2.5 g/kg plus 400 mg/kg paracetamol were found to have significantly (p<0.05) low levels of serum albumin (Table 2).
| Groups | ALB (mg/ml) | TBIL (μmol/L) | DBIL (μmol/L) | GGT (U/L) | AST (U/L) | ALT (U/L) |
|---|---|---|---|---|---|---|
| Control | 41.8 ± 3.90 | 2.49 ± 0.60 | 0.73 ± 0.34 | 0.94 ± 0.18 | 193.6 ± 21.65 | 104 ± 26.08 |
| Alc 2.5 g/kg | 37.07 ± 1.92 | 2.77 ± 0.66 | 1.28 ± 0.61 | 1.86 ± 0.68 | 319.60 ± 37.67 | 109.80 ± 14.36 |
| Alc 3.5 g/kg | 37.05 ± 2.24 | 3.19 ± 0.71 | 1.79 ± 0.40 | 2.14 ± 0.66 | 337.80 ± 34.97* | 118.60 ± 12.31 |
| Alc 4.5 g/kg | 31.77 ± 3.26* | 4.48 ± 1.40* | 3.06 ± 0.91* | 2.34 ± 0.80 | 451.20 ± 85.02* | 160.20 ± 29.02* |
| Para 40 mg/kg | 40.13 ± 3.42 | 2.59 ± 0.55 | 0.67 ± 0.14 | 1.76 ± 0.89 | 190.80 ± 40.80 | 111.00 ± 17.56 |
| Para 400 mg/kg | 32.64 ± 1.65* | 3.23 ± 0.65 | 2.21 ± 0.51* | 4.28 ± 1.76* | 357.20 ± 33.69* | 132.80 ± 33.66 |
| Alc 2.5 g/kg+Para 40 mg/kg | 35.70 ± 2.23 | 2.47 ± 0.17 | 096 ± 0.46 | 2.06 ± 1.02 | 249.40 ± 42.32 | 104.60 ± 11.41 |
| Alc 2.5 g/kg+Para 400 mg/kg | 33.21 ± 7.25* | 3.25 ± 0.35 | 1.35 ± 0.19 | 2.30 ± 1.11 | 332.80 ± 75.80* | 137.00 ± 31.21 |
| Alc 3.5 g/kg+Para 40 mg/kg | 40.32 ± 6.73 | 3.25 ± 0.25 | 1.58 ± 0.19 | 3.32 ± 1.73 | 324.60 ± 29.75 | 125.60 ± 12.69 |
| Alc 3.5 g/kg+Para 400mg/kg | 34.56±160 | 4.62 ± 0.70* | 2.00 ± 0.68* | 4.84 ± 1.50* | 321.40 ± 106.70 | 136.60 ± 33.89 |
| Alc 4.5 g/kg+Para 40 mg/kg | 34.48 ± 1.83 | 4.66 ± 1.37* | 2.77 ± 0.74* | 4.00 ± 1.71* | 378.00 ± 52.92* | 136.60 ± 20.38 |
| Alc 4.5 g/kg+Para 400 mg/kg | 34.08 ± 3.75 | 4.76 ± 1.02* | 3.10 ± 0.50* | 4.44 ± 1.21* | 484.20 ± 93.05* | 149.20 ± 15.32 |
The p value is for multiple comparisons between control and other treatments: * p<0.05. Para: Paracetamol; Alc: Alcohol; ALP: Alkaline Phosphatase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; GGT: Gamma Glutamyl transferase.
Table 2: Liver function tests.
Total and direct bilirubin were significantly (p<0.05) elevated in rats treated with 4.5 g/kg alcohol, 3.5 g/kg of alcohol plus 400 mg/ kg of paracetamol, 4.5 g/kg of alcohol plus 40 mg/kg and 4.5 g/ kg of alcohol plus 400 mg/kg of paracetamol. In the group treated with 400 mg/kg of paracetamol it was only direct bilirubin that significantly (p<0.05) elevated.
Gamma glutamyl transferase enzyme was significantly (p<0.05) elevated in the animals treated with paracetamol 400 mg/kg, 3.5 g/kg of alcohol plus 400 mg/kg of paracetamol, 4.5 g/kg of alcohol plus 40 mg/kg of paracetamol and 4.5 g/kg of alcohol plus 400 mg/kg of paracetamol.
Serum profiles of enzyme Aspartate Amino Transferase (AST) was significantly (p<0.05) elevated in rats treated with alcohol 3.5 g/kg and 4.5 g/kg, paracetamol 400 mg/kg, alcohol 2.5 g/kg plus paracetamol 400 mg/kg, 4.5 g/kg of alcohol plus 40 mg/kg paracetamol and 4.5 g/kg of alcohol plus 400 mg/kg. However, for Alanine Amino Transferase (ALT), it was only elevated in rats treated with alcohol 4.5 g/kg.
The critical AST/ALT ratio was calculated and it was observed that the control group had a value of 1.93 which was within the normal range of<2 (Figure 2).
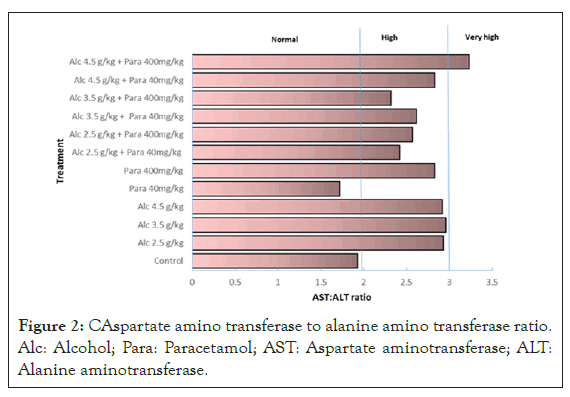
Figure 2: CAspartate amino transferase to alanine amino transferase ratio. Alc: Alcohol; Para: Paracetamol; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
The other group that had normal levels was that treated with 40 mg/kg of paracetamol. All the other groups had ratios above the normal values with the group treated with 4.5 g/kg of alcohol plus 400 mg/kg of paracetamol recording a very high ratio of 3.23.
Functions of kidney
Urea and creatinine levels in the control group were 8.71 mmol/L and 44.53 μmol/L respectively. In other groups the markers were stable except in those that received 4.5 g/kg of alcohol where urea levels was significantly (p<0.05) elevated to 11.25 mmol/L. While in the group co-treated with 4.5 g/kg of alcohol plus 400 mg/kg of paracetamol, urea and creatinine levels were significantly (p<0.05) elevated to 11.56 mmol/L and 77.01 μmol/L respectively.
Blood sugar levels
Four of the rat groups had glucose levels comparable to the control group (p>0.05). However, those treated with 3.5 g/kg alcohol, 4.5 g/kg alcohol, 3.5 g/kg alcohol plus 40 mg/kg paracetamol and 4.5 g/kg of alcohol plus 40 mg/kg of paracetamol had glucose levels that were significantly (p<0.05) lower than those of the control group. While those that were treated with 2.5 g/kg of alcohol plus 400 mg/kg of paracetamol; and 4.5 g/kg of alcohol plus 400 mg/kg of paracetamol had glucose levels that were significantly (p<0.05) higher than those of the control group.
Liver histopathology
Control group and those treated with 40 mg/kg paracetamol showed normal renal tissue. All other treatments showed pathology of cytoplasmic vacuolization and infiltration by inflammatory cells. Steatosis and hepatocellular necrosis was observed in most treatment groups except those that received alcohol at doses of 2.5 and 3.5 g/kg (Table 3).
| Groups | Cytoplasmic vacuolization | Infiltration of inflammatory cells | Steatosis | Hepatocellular necrosis |
|---|---|---|---|---|
| Control | - | - | - | - |
| Alc 2.5 g/kg | + | + | - | - |
| Alc 3.5 g/kg | + | + | - | - |
| Alc 4.5 g/kg | + | + | + | + |
| Para 40 mg/kg | - | - | - | - |
| Para 400 mg/kg | + | + | + | + |
| Alc 2.5 g/kg+Para 40 mg/kg | + | + | + | + |
| Alc 2.5 g/kg+Para 400 mg/kg | + | + | + | + |
| Alc 3.5 g/kg+Para 40 mg/kg | + | + | + | + |
| Alc 3.5 g/kg+Para 400 mg/kg | + | + | + | + |
| Alc 4.5 g/kg+Para 40 mg/kg | + | + | + | + |
| Alc 4.5 g/kg+Para 400 mg/kg | + | + | + | + |
Alc: Alcohol; Para: Paracetamol.
Table 3: Liver histopathology.
Kidney histopathology
Histological features of the control group indicated normal tissue devoid of pathology. All treatment groups showed cellular infiltration and cytoplasmic vacuolization, but the latter was absent in rats that received 40 mg/kg of paracetamol. Rats that were treated with 400 mg/kg of paracetamol and combinations of alcohol and paracetamol had patches of cellular necrosis except the group treated with 4.5 g/kg alcohol plus 400 mg/kg paracetamol. While those treated with 400 mg/kg paracetamol, 2.5 g/kg alcohol plus 400 mg/kg paracetamol, 3.5 g/kg alcohol plus 400 mg/kg paracetamol and 4.5 g/kg alcohol plus 400 mg/kg paracetamol in addition had sinusoid formation (Table 4).
.| Groups | Cytoplasmic vacuolization | Infiltration of inflammatory cells | Sinusoid formation | Necrosis |
| Control | - | - | - | - |
| Alc 2.5 g/kg | + | + | - | - |
| Alc 3.5 g/kg | + | + | - | - |
| Alc 4.5 g/kg | + | + | - | - |
| Para 40 mg/kg | - | + | - | - |
| Para 400 mg/kg | + | + | + | + |
| Alc 2.5 g/kg+Para 40 mg/kg | + | + | - | + |
| Alc 2.5 g/kg+Para 400 mg/kg | + | + | + | + |
| Alc 3.5 g/kg+Para 40 mg/kg | + | + | - | + |
| Alc 3.5 g/kg+Para 400 mg/kg | + | + | + | + |
| Alc 4.5 g/kg+Para 40 mg/kg | + | + | - | + |
| Alc 4.5 g/kg+Para 400 mg/kg | + | + | + | - |
Alc: Alcohol; Para: Paracetamol.
Table 4: Kidney histopathology.
This study compared the effects of individual and combined treatments of alcohol and paracetamol on the biological parameters of a rat model. Individually, alcohol (≥ 3.5 g/kg) and paracetamol (400 mg/kg) caused listlessness, rough hair coat and poor appetite in the study animals. These results are consistent with those of Ning et al. [25] and Juma et al. [26]. Review of literature shows no information on the effects of combined use of alcohol and paracetamol on physical features of animal model. This work shows that when the two drugs were used together they had similar effects on morphological features as the individual treatments. Factors that affect morphological characteristics of rodents include climate, sickness, distress and pain [27,28]. Here, the subtle changes in these features is likely due to sickness, distress or pain induced by the drugs.
Multiple comparisons of the control and treated groups showed that initially alcohol had insignificant effect on body weight but on the fourth week there was significant weight gain. Previous studies on the impact of alcohol on body weight are contentious with some reports showing a positive correlation [29] and others an inverse association [30,31]. The weight gain reported here is indicative of positive energy balance due to the calorific effect of ethanol. The mechanism through which alcohol may modify energy balance and subsequently body weight, include impact on nutrient digestion and absorption, interference with lipid oxidation, thermogenesis, and enhanced adenosine triphosphate breakdown [32].
Paracetamol had insignificant effect on the body weight except for the 40 mg/kg group that recorded weight gain in week four. This has not been previously reported for the drug and further research is required to establish whether the weight gain is drug related or an isolated event. When administered together alcohol and paracetamol showed initial weight loss that was reversed to weight gain in week four. This implies that simultaneous use of the drugs can result to either an energy surplus or deficit, and the latter is likely due to the effects of alcohol.
In the current study, alcohol and paracetamol had no significant effect on the hematological profile. Previous studies show inconsistent findings with some reporting increase [33], decrease [34] or no effect [35,36]. Similar mixed results have also been reported with paracetamol [37-40]. Disagreement in results between studies could be attributed to factors such as the amount and duration of alcohol treatment and the experimental model employed. Changes in hematological profiles is an indication of interference with the bone marrow and the immune system. It can therefore be concluded that at the investigated doses, alcohol and paracetamol, did not influence the activity of the two systems.
The most important clinical manifestation of chronic alcohol abuse is alcoholic liver disease. The condition is characterized by steatosis, hepatitis and cirrhosis [41]. Due to cellular damage, elevated liver enzymes is a common scenario among patients suffering from liver disease [42]. Here Aspartate Amino Transferase (AST) was high in animals treated with 3.5 g/kg alcohol while those given 4.5 g/kg had elevated AST and alanine aminotransferase (ALT). The elevated enzymes, reduced albumin, and increased bilirubin is a further indication of liver injury, a phenomenon that was supported by histopathological data. These results reaffirms that alcohol is hepatotoxic and the effect was dose dependent. Interestingly, aspartate aminotransferase/alanine aminotransferase ratio was higher than the cut off value of 2 in all alcohol treatments. This implies that the index is a more predictive biomarker tool for alcohol exposure and hepatic injury. A result also consistent with the histopathology results of the liver sections.
At 400 mg/kg dose, paracetamol caused elevation of Gamma Glutamyl Transferase (GGT) and AST. The fact that gamma glutamyl transferase was only elevated with the high dose of paracetamol and not alcohol is an indication that the former was more hepatotoxic. This could be due to differences in the median lethal dose (LD50) and mechanisms of toxicity of the drugs. For alcohol, the LD50 in rat is 7060 mg/kg (LHS, 2004) and hepatocytes injury is caused by oxidative stress due to enhanced generation of reactive oxygen species and depletion of antioxidant defense system [43]. For paracetamol the LD50 is>4000 mg/kg [44] and cellular damage is due to lipid peroxidation induced by the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) [45].
When alcohol and paracetamol were administered separately at 4.5 g/kg and 40 mg/kg, respectively, the GGT levels were normal, but the enzyme levels were elevated when the drugs were administered together. Besides, that 4.5 g/kg of alcohol and 400 mg/kg of paracetamol, had AST/ALT ratio that was>3, a value that is indicative of advanced liver injury [46]. These results shows that combined use of paracetamol and alcohol had more extensive liver damage and this was independently supported by histopathological analysis. Toxicity due to the combined use of the drugs can be attributed to the fact that alcohol reduces glutathione content thus reducing the margin of safety of paracetamol [47]. This means that chronic and excess use of paracetamol in management of hangover among heavy users of alcohol can increase the risk of liver disease. On the other hand moderate dosages of the drugs are well tolerated and they therefore exhibit a lower risk of liver disease.
Assessment of renal function showed that paracetamol did not affect serum urea and creatinine but 4.5 g/kg alcohol caused uremia. High blood urea is a consequence of several conditions such as kidney disease, blocked urinary tract, high protein diets, congestive heart failure and dehydration [48-51]. In this experimental model, it is reasonable to conclude that the uremia is pathologically linked to the alcohol induced renal injury.
Some reports suggest that paracetamol at therapeutic doses is safe and effective even in chronic alcoholics [52,53]. However, in another report the drug can cause kidney diseases in patients with alcohol dependency. In the current work simultaneous use of the two drugs resulted in significant elevation of urea and creatinine in animals treated with 4.5 g/kg of alcohol and 400 mg/kg of paracetamol. Histopathological results showed more renal pathology when both drugs were used together than when used individually. Increased toxicity can be explained from the observation that interaction of alcohol and paracetamol result in the increased production of NAPQI, the highly toxic metabolite of paracetamol [54]. These results therefore suggest that chronic use of paracetamol among heavy users of alcohol increases the risk of kidney disease.
Experimental animals treated with paracetamol had normal levels of blood glucose, while those given 3.5 g/kg and 4.5 g/kg of alcohol displayed low sugar levels. Depending on the circumstance alcohol can cause hypoglycemia [55] or hyperglycemia [56] and this is because of its influence over insulin and glucagon, both of which are the hormones involved in glucose counter-regulation [57].
Liu et al. reported that low to moderate doses of alcohol have a protective effect against diabetes, while Vonghia et al. [58] found that acute alcohol intoxication can elevate blood glucose. For paracetamol the drug can normalize increased blood glucose [59] but it is also associated with hyperglycemia [60]. This study and others show that the effects of alcohol or paracetamol on blood sugar is influenced by factors such as dosage and experimental design. This work further suggests that neither of the drugs has a predominant effect over the other in modulating blood glucose.
It can be concluded that both alcohol and paracetamol did not affect the hematological profile and by extension they had no influence on the activity of the bone marrow and the immune system. Furthermore, moderate doses of paracetamol is safe, but high doses of the drug and chronic use of alcohol is hepatotoxic. It also shows that combined use of the drugs increased the risk of liver injury. Also that AST/ALT index is a more predictive tool for alcohol exposure and liver injury. However, considered individually, alcohol and paracetamol have a low risk of renal damage but when used together the risk is increased.
This study recommends the use of AST/ALT index, instead of the individual values, as the biomarker tool for alcohol exposure and hepatic injury. Equally, this study does not support misuse of paracetamol among heavy users of alcohol as there is increased risk of hepatic and renal diseases.
Further studies however may be performed on dose response experiments to clarify whether combined use of alcohol and paracetamol causes positive or negative energy balance. Above all, it will also be important to establish the sensitivity range of the AST/ALT index as a biomarker tool of alcohol exposure and hepatic injury.
This study acknowledges the following people involved in this study: Mr. Francis Wanyama, Department of Human pathology, University of Nairobi; Mr. Joseph Kihara, Department of Human Anatomy, Kenyatta University; Mr. Kelvin Juma, Mr. Eliakim Mbaka, Mr. Daniel Gitonga, and Mr. James Ngunjiri, Department of Biochemistry, Microbiology and Biotechnology, Kenyatta University for their technical support in this study.
Citation: Oloo Q (2021) The Effects of Paracetamol on the Liver and Kidney Functions of a Rat Model Following Prolonged Alcohol Administration. J Drug Metab Toxicol. 12:256.
Received: 17-May-2021 Accepted: 31-May-2021 Published: 07-Jun-2021 , DOI: 10.35248/2157-7609.21.12.256
Copyright: © 2021 Oloo Q. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.