Research Article - (2024)Volume 8, Issue 2
Background/Objectives: The non-alcoholic fatty liver disease is a common cause of chronic liver disease which associated with obesity and diabetes and has a worldwide outbreak. The aim of this study cytokines and adipokines caused by NAFLD.
Methods: Thirty-five Wistar male rats after establish fatty liver models by feeding a high fat diet (HFD, 12 weeks) were randomly divided into five groups (n=7 rats/each group); control (standard diet), VEH (HFD+VEH), high dose Shilajit (HFD+250 mg/kg/day Shilajit by gavage for 2 weeks), low dose Shilajit (HFD+150 mg/kg/day Shilajit by gavage for 2 weeks), pioglitazone (HFD+10 mg/kg/day pioglitazone by gavage for 2 weeks). The serum levels of glucose, insulin, IL-1β, IL-6, TNF- α, IL-10, adiponectin and resistin were measured after the two-weeks of intervention.
Results: The results showed that in VEH group the serum levels of glucose (p<0.001), insulin (p<0.001), IL-1β (p<0.05), IL-6 (p<0.05), TNF-α (p<0.05) and resistin (p<0.01) significantly increased, while IL-10 (p<0.001) and adiponectin (p<0.01) levels decreased in comparison to the control group. However, treatments with high and low doses of Shilajit as well as pioglitazone significantly decreased insulin resistance (p<0.001), serum levels of glucose (p<0.001), IL-1β (p<0.01), TNF-α (p<0.01) and resistin (p<0.01) while increased IL-10 (p<0.001) and adiponectin (p<0.01) in comparison to VEH group.
Conclusion: The study showed that Shilajit by modulating the serum levels of cytokines, adipokines and reducing of Insulin resistance index can improve NAFLD.
NAFLD; Shilajit; HFD; Adipokines; Cytokines; Glucose
Non-Alcoholic Fatty Liver Disease (NAFLD) is the common cause of chronic liver diseases caused by fat accumulation in the liver due to the high intake of fat and calories. This disease occurs in the absence of alcohol consumption. Globally, the incidence of NAFLD is estimated to be around 25%-30%. The high prevalence and chronic nature of this disease affect the quality of life of patients and impose an economic burden on society [1].
In this disease, high fat accumulation in the liver cells disrupts the normal activity of the liver tissue, leading to a progressive course ranging from simple steatosis to Non-Alcoholic Steatohepatitis (NASH) and end-stage liver diseases such as fibrosis, cirrhosis and carcinoma. The development and progression of NAFLD are highly linked to the features of metabolic syndrome including high blood glucose, insulin resistance, dyslipidemia, obesity and diabetes. However, some studies reported that NAFLD may develop in non-obese patients and individuals without metabolic syndrome.
One of the mechanisms known to develop and promote fatty liver is insulin resistance; Also, the correlation between insulin resistance and NAFLD has been reported in several studies. Insulin resistance assessment by Homeostasis Model of Insulin Resistance Index (HOMA-IR), was initiated by Matthews, et al.
Zhengjun, et al. showed that HOMA-IR was one of the risk factors of NAFLD. The adipose tissue (especially visceral) is capable of producing and secreting several bioactive proteins and adipokines, which play a central role in regulating the metabolism of liver and peripheral glucose and lipid. Among them, inflammatory cytokines including Tumor Necrosis Factoralpha (TNF-α), Interleukins (IL-1β, IL-6, and IL-8) are involved in insulin resistance as a key component of NAFLD. Clinical and experimental studies have reported a direct correlation between the increased pro-inflammatory cytokines and the development of fatty liver disease [2].
Adipose tissue also produces adipokines such as adiponectin and resistin. A great number of studies have reported that adiponectin and resistin or other adipokines can influence NAFLD. The studies have shown that low serum levels of adiponectin are associated with NAFLD. In a study done by Raika Jamali, et al. in 2016, it was reported that certain adipokines could accurately determine the severity of NAFLD histology. Currently, one of the most important issues in understanding the pathology of NAFLD is chronic inflammation, which plays an important role in the progression and severity of the disease. Chemokines and also adipokines can be considered as factors that play an important role in the inflammation and promotion of fatty liver, so they can be considered as therapeutic targets.
Recent studies have focused on the potentiality of herbal medicines as a therapeutic strategy for the prevention and treatment of NAFLD, due to their effectiveness and low side effects. Shilajit, also named mineral pitch, is a sticky blackishbrown substance which is mainly found in the steep rocks of Himalayas mountains that sinks out of the cliffs during the warm months of the year Shilajit contains mainly humic substances functionally divided into Fulvic Acid (FvA), folate, Humic Acid (HA) and hymen acid, which are responsible for its biochemical activities. It also contains vitamin A, B, C, terpenoids, polyphenol complexes, phospholipids and many essential microelements such as iron, zinc, magnesium, nickel, cobalt, copper, manganese and chrome. In addition, Shilajit had significant anti-inflammatory effects in chronic inflammation. Also, Shilajit can significantly decrease carrageenan-induced edema in rat’s paw. It has also been reported that Shilajit has anti-allergic effects on histamine release [3].
Considering that the inflammatory and oxidant agents are involved in the development of non-alcoholic fatty liver and Shilajit had anti-inflammatory and antioxidant properties, it is expected that Shilajit can improve the symptoms of the disease. In addition, little is known about the mediating factors and the molecular mechanism of Shilajit effects in NAFLD. Therefore, we supposed that one of the action mechanisms of Shilajit protection could be changing the level of cytokines and adipokines considering the anti-inflammatory effect of Shilajit and this hypothesis was examined in this study.
Animals
Thirty-five male Wistar rats, weighing 160 ± 20 g, were purchased from the experimental animal center of Kerman university of medical sciences (Kerman, Iran). Animals were housed 5 per cage and kept in a temperature and humiditycontrolled room (20°C ± 3°C temperature, 45%-55% humidity) on a 12 h light-dark cycle and allowed water and diet ad libitum. This study was approved by the approval of the medical ethics committee of Kerman university of medical sciences (Approval No: IR. KMU. REC. 1394. 681). Animals were treated in accordance with the guide for the care and use of laboratory animals (8th edition, National academies press).
Study design
Following one week period of adjustment, animals after establish fatty liver model by feeding a High-Fat Diet (HFD, 12 weeks (by examining the status of the liver, histologically and biochemically in pilot test, which the results of it are being under the publication's status, were divided randomly into five groups as follow (n=7 in each group): Control (standard diet), Vehicle (VEH) group: Received distilled water as the vehicle; Low-dose Shilajit (L.Sh) group: Received 150 mg/kg/day Shilajit; High-dose Shilajit (H.Sh) group: Received 250 mg/kg/day Shilaji; And Pioglitazone Group (Pio): received10 mg/kg/day pioglitazone. Each group received their respective treatments for 12 days by gavage after the induction of NAFLD model. In this study, doses of Shilajit have been used whose antiinflammatory effects have been reported in previous studies [4].
Shilajit preparation
Shilajit was prepared from the local residents of Sardoiyeh in Jiroft (Kerman, Iran) with the approval of the faculty of pharmacy. It was dried following at least 2-3 times of washing and then powdered and dissolved in normal saline and positioned on a shaker for 24 h. Then, it was passed through a clean filter to remove the possible impurities. Subsequently, the solution was centrifuged at 5000 g for 10 min and then sterilized in the autoclave. During treatment, the suspension was prepared every day with the required concentration [5].
High-fat diet preparation
An HFD containing 20% fat (170 g animal fat+30 g corn oil), 1% cholesterol, 0.5 % bile salt and 10% sucrose were added to the normal rat chow. The HFD was prepared weekly in a pellet form and refrigerated until use.
Blood serum preparation
At the end of the treatment, all rats were sacrificed following 12 h fasting under deep anesthesia with pentobarbital sodium (50 mg/kg, i.p). Blood samples were collected via heart puncture and then centrifuged at 4000 rpm for 10 min at 4°C to obtain the serum. The serum samples were allocated into tubes and stored at -70 °C for further biochemical assessments [6].
Biochemical analyses
Blood glucose levels were determined using an autoanalyzer. Insulin levels in the serum samples were quantified by an Enzyme-Linked Immunosorbent Assay (ELISA) kit. HOMA-IR was calculated using the following formula: HOMA-IR=fasting plasma glucose (mg/dl) × fasting plasma insulin (μU/ml) /405.
Measurements of cytokines and adipokines
Serum levels of resistin, adiponectin, pro-inflammatory cytokines including TNF- α, IL-6 and IL-1β, as well as IL-10, an anti-inflammatory cytokine, were also measured using commercially available ELISA kits. according to the manufacturers' instructions [7].
Statistical analysis
The data were analyzed using SPSS software version 20. First, the normality of the data was assessed by the Kolmogorov- Smirnov test.
The results of the first experiment were analyzed using an independent sample t-test and one-way Analysis of Variance (ANOVA) followed by Tukey’s post-hoc test was used for multiple comparisons in the second experiment. Data are reported as means ± SEM. P-value<0.05 was considered statistically significant.
Confirmation of fatty liver induction
As shown in Table 1, the 12 weeks feeding with HFD significantly (p<0.05) increased serum levels of liver enzymes such as AST and ALT, as well as serum levels of TC, TG and VLDL in the VEH group. In addition, HFD significantly (p<0.05) increased hepatocyte steatosis in the VEH group as compared to the control group [8].
| Groups | TG (mmol/l) | TC (mmol/l) | VLDL (mmol/l) | AST (U/L) | ALT (U/L) | Steatosis (mean/SEM) |
|---|---|---|---|---|---|---|
| Control | 77.12 ± 5 | 67.05 ± 4 | 15.42 ± 0.9 | 165 ± 8 | 72 ± 2 | 0.2 ± 0.02 |
| Vehicle | 102 ± 6* | 99.5 ± 5* | 20.4 ± 1.3* | 223 ± 20** | 194 ± 29** | 2.6 ± 0.2** |
Table 1: Serum lipid levels, hepatic enzymes and steatosis of rats with fatty liver induced by 12 weeks of high-fat diet.
Moreover, the results of the histological study confirmed induction of fatty liver. As shown in Figure 1, in the control group receiving standard diet the liver lobules, were distinct and the regular cell cords were arranged, while in the VEH group typical steatosis was associated with cytological ballooning and mild to moderate lobular inflammatory cell infiltration [9].
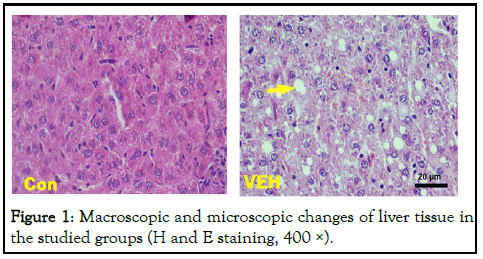
Figure 1: Macroscopic and microscopic changes of liver tissue in the studied groups (H and E staining, 400 ×).
The serum level of glucose, insulin and HOMA-IR
The results of biochemical assessments in the current experiment revealed that VEH group had higher serum levels of glucose (p<0.001) and insulin (p<0.001) than the control group. However, treatment with low and high doses of Shilajit as well as pioglitazone significantly (p<0.001) decreased serum glucose levels in the VEH animals. Although serum insulin levels did not change in the L.Sh and H.Sh groups, it was significantly decreased in the Pio group (p<0.05) as compared to the VEH group (Figure 2).
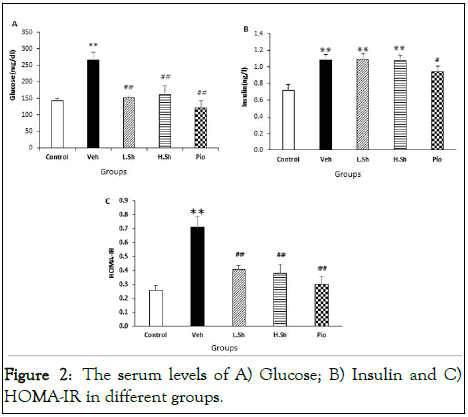
Figure 2: The serum levels of A) Glucose; B) Insulin and C) HOMA-IR in different groups.
The results also showed that HOMA-IR was significantly (p<0.001) increased in the VEH group as compared to the control animals, indicating the development of insulin resistance. Nevertheless, administration of Shilajit and pioglitazone significantly (p<0.001) decreased HOMA-IR as compared to the VEH group (Figure 3).
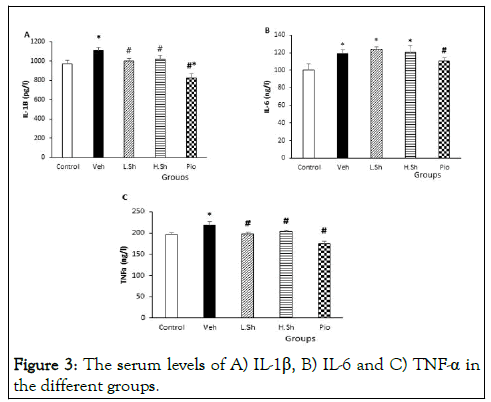
Figure 3: The serum levels of A) IL-1β, B) IL-6 and C) TNF-α in the different groups.
Serum levels of IL-1β, IL-6 and TNF-α
Our results also demonstrated that induction of fatty liver significantly increased serum levels of IL-1β, IL-6 and TNF-α as compared to the control group (p<0.05). Serum levels of TNF-α and IL-1β were significantly (p<0.01) reduced in the L.Sh, H.Sh and Pio groups compared to the VEH group (p<0.01). Although administration of pioglitazone significantly decreased serum levels of IL-6 (p<0.05), there were no significant changes in the IL-6 levels in the Shilajit-received groups compared to the VEH group [10].
The serum levels of IL-10
The results also showed that serum levels of IL-10 were significantly (p<0.001) decreased in the VEH with compared to the control group. Only administration of the low dose of Shilajit significantly (p<0.001) increased IL-10 serum levels compared to the VEH group (p<0.01). However, but there were no significant changes in the IL-10 levels in the H.Sh and Pio groups compared to the VEH group (Figure 4).
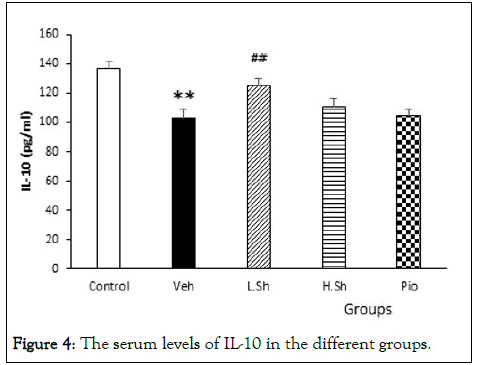
Figure 4: The serum levels of IL-10 in the different groups.
The serum levels of resistin and adiponectin
The results of ELISA also revealed that serum levels of resistin were significantly increased in the VEH group as compared to the control group (p<0.01). Moreover, the VEH group had lower adiponectin serum levels than the control group (p<0.01). However, treatments with Shilajit and pioglitazone significantly reduced serum resistin levels, while increased adiponectin levels in the serum compared to the VEH group (p<0.01) (Figure 5).
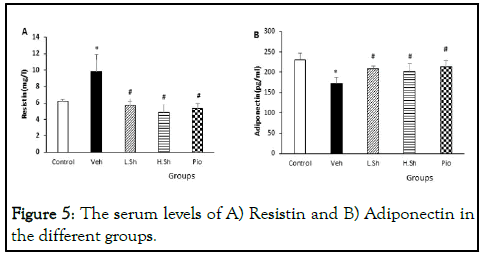
Figure 5: The serum levels of A) Resistin and B) Adiponectin in the different groups.
The present study was performed to investigate some molecular mechanism of Shilajit in the non-alcoholic fatty liver disease. The results of the present study indicate that giving Shilajit after NAFLD induction, resulted in reducing serum levels of glucose, insulin, insulin resistance index (HOMA-IR), IL-1β, TNF-a and resistin, in addition, increases IL-10 and adiponectin. These results were inaccordance with previous report of Bhattacharya, et al. Who foundthat that oral administration of Shilajit attenuated glucose in diabetic rats; also observed that Shilajit boosted the hypoglycemic action of insulin [11].
There are some documents showing that Shilajit increases superoxide dismutase, catalase and glutathione peroxidase activities in rats. Shahrokhi, et al reported that treatment with Shilajit prevents acetic acid-induced colitis in rats and this protective effect may be due to its antioxidant and antiinflammatory actions. In some scientific reports, it has also been described that Shilajit has anti-allergic effects on histamine release. We also show that Shilajit has an obvious protective effect against brain edema and liver injury due to its antioxidant and anti-inflammatory actions.
The important role of cytokines in the pathophysiology of NAFLD has been reported in numerous studies. Contrary to the physiological conditions in which cytokines are found to be very low in liver cells, pathological conditions such as accumulation of fat in the liver cells can increase the production of inflammatory cytokines, playing an important and active role in the development and promotion of the nonalcoholic liver disease. IL-1β, IL-6 and TNF-α are among the pro-inflammatory cytokines contributing to the development of fatty liver disease. Research has also shown that IL-1β is involved in the development of the fatty liver disease stimulates the accumulation of cholesterol and triglycerides and lipid droplet formation in the liver.
Consistent with the results from this study, in which the levels of adiponectin and resistin in the fatty liver group (VEH) decreased and increased, respectively, the expression of resistin and adiponectin was measured in the adipose tissue, respectively, increased and decreased in NAFLD patients; a positive correlation was also found between resistance and the inflammatory response in NAFLD patients. Consistent with the results from this study, Ghazi et al showed Shilajit treatment reduced IL-6, IL-1β and TNF-α levels, following hepatic injury induced by administration of a single dose of acetaminophen.
It is supposed that the main biological effects of Shilajit depend on the presence of Fulvic Acid (FDA), Humic Acid (HA) and dibenzo-a-pyrones, which are carrier molecules for active components. Several studies indicate that FvA can act as an antiinflammatory by reducing the release of proinflammatory mediators from cells. Show that FvA can reduce Tumor Necrosis Factor alpha (TNF-α) expression after exposure to the endotoxin Lipopolysaccharide (LPS) in differentiated human monocytes. In the several studies have demonstrated anti-inflammatory and antioxidant effects of HA. Have shown that humic potassium reduces serum cytokines levels of pro-inflammatory cytokines like IL-1β, IL-6 and TNF-α, as produced by mononuclear cells. The results of this study indicate that shilajit hepatoprotective effects probably is due to the reduction of anti-inflammatory cytokines and the reversal increase of pro-inflammatory cytokines and adipokines modulation after NAFLD. However, further studies are required to determine another functional mechanism of Shilajit [12].
The results indicated that Shilajit could play a positive role in reducing insulin resistance, improving diabetes and lowering blood glucose levels after NAFLD. Also, further analysis showed the modulation effects of Shilajit on cytokines and adipokines; these included the reduction of inflammatory cytokines, the increase of anti-inflammatory cytokines, the decrease of resistin and the rise of adiponectin. It is possible that Shilajit can be used as a complementary drug in the treatment of non-alcoholic fatty liver disease. Further studies, such as clinical trials, are recommended for the purpose of using Shilajit in human diseases treatment.
None of the authors have any conflict of interest.
This study is supported by Kerman university of medical sciences, Kerman, Iran.
The present study was financially supported by physiology research center, Kerman university of medical sciences, Kerman, Iran. This work was part of the Ph.D. thesis of Miss Ghezelbash at the department of physiology.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ghezelbash B, Shahrokhi N, Khaksari M, Asadikaram G, Shirazpour S (2024) The Effects of Shilajit on Serum Levels of Cytokines and Adipokines in Rats with the Non-Alcoholic Fatty Liver Disease. J Pharma Reports. 8:212.
Received: 22-Jul-2020, Manuscript No. jpr-24-5526; Editor assigned: 28-Jul-2020, Pre QC No. jpr-24-5526 (PQ); Reviewed: 11-Aug-2020, QC No. jpr-24-5526; Revised: 01-Jun-2024, Manuscript No. jpr-24-5526 (R); Published: 28-Jun-2024 , DOI: 10.35248/jpr.24.8.212
Copyright: © 2024 Ghezelbash B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This study is supported by Kerman university of medical sciences, Kerman, Iran.