Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research - (2020)Volume 11, Issue 6
Aim: To evaluate the efficacy and safety of the new lipid tear substitute VisuEvo® in patients with dry eye disease (DED).
Methods: 19 patients with evaporative or iatrogenic DED were enrolled and evaluated at baseline, week 2 and week 6. After baseline, they were instructed to self-administer VisuEvo three times daily for the whole study duration. Tear break-Up Time (TBUT), Schirmer I, Ferning, osmolarity, cytokine and lipid expression, ocular surface staining, patient satisfaction, and OSDI score were measured.
Results: During the study, TBUT progressively increased from 3.0 ± 1.9 sec at baseline to 6.4 ± 1.7 sec at final visit (P<0.0001), and OSDI progressively decreased from 39 ± 12 at baseline to 20 ± 15 at final visit (P<0.0001). Osmolarity significantly reduced from 328 ± 14 mOsm/L at baseline to 306 ± 14 mOsm/L at final visit (P=0.03). A progressive reduction of cytokine and lipid expression was shown, being significant for IFN-ˠ (P=0.01) and sphingosine (P=0.01). No changes were shown for Schirmer Test, conjunctival and corneal staining. Safety profile was excellent as no adverse events occurred; patients were highly satisfied by treatment.
Conclusion: VisuEvo is an effective and safe option for DED management.
E vaporative dry eye disease; Tear Break-Up Time (TBUT); Ocular surface disease index questionnaire; Meibomian gland disturbance; Preservatives; Ocular surface
Dry Eye Disease (DED) is a multifactorial disease characterized by loss of homeostasis of the tear film, subclinical ocular surface (OS) inflammation, and neurosensory abnormalities leading to ocular symptoms [1]. The high prevalence of DED (higher than 20% on an adult Caucasian population) [2] together with the high frequency of symptoms [2] make it one of the most common causes for seeking an ophthalmologic evaluation [3].
DED can be due to Meimobian Gland Disturbance (MGD), endocrine disregulation, abnormal immune response, or exposure to the chronic toxic effect of preservatives (as in glaucoma management). As a consequence DED may show tear deficiency, increased evaporation or mixed components. Due to the very high frequency of MGD, increased evaporation is believed to be the most common form of DED overall [4]. Tear supplementation is of key importance for DED treatment, in order to increase or stabilize tear film. Among supplements, lipid-containing eye drops are growing in both availability and popularity, primarily due to the increased attention paid to hyper-evaporation in the pathogenesis of DED [5]. VisuEvo® (Visufarma SpA, Italy) is a new multidose, non-preserved ophthalmic solution consisting on a ultrafiltered liposomal nano-dispersion associated with vegetable oil rich in Omega 3 (docosahexaenoic acid- DHA and eicosapentaenoic acid – EPA), Vitamin D3 and Vitamin A palmitate. VisuEvo is supposed to be primarily effective in evaporative forms of DED and, thanks to Vitamin A, also on DED associated with mucin impairment.
The aim of this study is to report the efficacy of VisuEvo on a group of patients with DED.
In this paper we report the efficacy and safety data of VisuEvo, as evaluated onto a multicenter, double-blind, randomized, prospective cross-over study, registered at www.clinicaltrial.gov (identifier: NCT03833882) and approved by Comitato Etico Milano Area 1; the study followed the Tenets of the Declaration of Helsinki and informed consent was obtained from participants [6,7]. In this paper, we used the prospective, interventional data derived from the previous study without any modification or adjunction. We analysed the first 6 weeks of treatment with VisuEvo (period 1 of the cross-over design) of patients from the Eye Clinic, ASST Santi Paolo Carlo, San Paolo Hospital, University of Milan, Milan, Italy. 19 consecutive patients with DED fulfilling the inclusion and exclusion criteria were included. Main inclusion criteria were: TBUT<7 seconds, OSDI score of 13 or more, Schirmer I test>10 mm at 5 minutes; patients falling in one of the following groups: active obstructive MGD, high tear film evaporation, females in menopause, glaucomatous patients receiving one or more treatments preserved with benzalkonium chloride for at least 2 years and showing an abnormal Ferning test (Types 3 or 4 according to Rolando et al. [8]). Main exclusion criteria were: coexisting OS diseases other than DED, autoimmune diseases, Sjogren syndrome, history of corneal trauma, use of contact lenses, any eye surgery performed 3 months before inclusion, inability to self-administer study medications and known allergic sensitivity to any of the device ingredients.
Data shown in this paper were measured at baseline, at week 2, and week 6. A screening visit was performed one week before baseline; at that time patients stopped any eventual lubricating treatment. After baseline to week 6, patients self-administered one drop of VisuEvo into conjunctival sac three times daily.
At each visit, patients underwent the following tests in the following order: Ocular Surface Disease Index questionnaire (OSDI, Copyright 1995, Allergan Inc, Irvine, California, US) [9], measurement of blinking and of osmolarity, TBUT, fluorescein staining, lissamine staining, Schirmer I test. Tear Ferning test, tear sampling and cytokine and lipid expression were measured at baseline and at week 6. The following tests were performed just on subgroups of patients chosen at random: measurement of blinking (15 patients), osmolarity (6 patients), tear sampling for cytokine and lipid measurement (11 patients). Ferning test was performed on the 4 glaucoma patients. OSDI questionnaire was administered to the patient by a nurse who helped in filling in the answers without interfering on patient’s judgement. OSDI score ranges from 0 to 100 (0-12, normal; 13-22, mild DED; 23- 32, moderate DE; 33 or more, severe DED).
Outcome assessments
The primary objective was evaluating the efficacy of VisuEvo in improving tear film stability (TBUT increase in seconds) over the course of the study. The secondary outcomes were improvements of symptoms (OSDI score), and of inflammatory parameters. Also safety was assessed by monitoring patients’ satisfaction and the occurrence of adverse events (in particular blurring) during the study.
Statistical analysis
In this paper, we used the dataset from a previous study without any modification or adjunction. The previous study lasted 12 weeks with a cross-over shift after week 6; patients had a 1-week washout from DED medications before baseline; washout was absent after cross-over had occurred [7]. As the aim of this study was to evaluate the efficacy of VisuEvo compared with baseline, we extrapolated from the dataset just data from those patients using VisuEvo on the first 6-week period from our site. Both eyes of each patient, if eligible, were tested and treated. The analyses were then performed only on the worse eye per patient based on TBUT or, in case of identical TBUT value, on the right eye. All study variables had a normal distribution as verified with the Kolmogorov-Smirnov test. Data were analysed using twoways t-test for paired data; p<0.05 was considered statistically significant.
Nineteen subjects were enrolled in the study; age was 59 ± 21 years; 68% were female. All patients concluded the 6-week study period. 5 patients fell into each of the following group: active obstructive MGD, high tear film evaporation, females in menopause; 4 were glaucomatous patients.
The results of the study are summarized in Table 1. In the course of the study TBUT, OSDI and osmolarity improved at each visit compared with baseline, whereas negligible changes were shown for Schirmer test, ocular surface staining and blinking (apart from a borderline statistically significance for incomplete blinking at week 6).
| Baseline | Week 2 | p | Week 6 | p | |
|---|---|---|---|---|---|
| Tear Break-up Time (sec) | 3.0 ± 1.9 | 4.6 ± 1.9 | 0.01 | 6.4 ± 1.7 | <0.0001 |
| OSDI questionnaire score | 39 ± 12 | 22 ± 17 | 0.03 | 20 ± 15 | <0.0001 |
| Schirmer Test (mm/5 min) | 16 ± 5 | 15 ± 8 | 0.78 | 17 ± 10 | 0.65 |
| Corneal staining | 0.4 ± 0.6 | 0.2 ± 0.4 | 0.1 | 0.3 ± 0.5 | 0.44 |
| Conjunctival staining | 0.8 ± 0.7 | 0.6 ± 0.6 | 0.29 | 0.6 ± 0.6 | 0.3 |
| Osmolarity (mOsm/L) | 328 ± 14 | 303 ± 17 | 0.02 | 306 ± 14 | 0.03 |
| Incomplete blinks (n/min) | 7 ± 6 | 7 ± 5 | 0.48 | 4 ± 3 | 0.07 |
| Complete blinks (n/min) | 18 ± 13 | 17 ± 11 | 0.59 | 19 ± 16 | 0.3 |
| Total blinks (n/min) | 25 ± 12 | 24 ± 15 | 0.4 | 23 ± 15 | 0.35 |
| IL-6 (pg/mL) | 11.3 ± 11.0 | n/p | n/a | 6.4 ± 9.0 | 0.29 |
| IL-8 (pg/mL) | 644 ± 753 | n/p | n/a | 444 ± 671 | 0.55 |
| IFN-γ (pg/mL) | 2.9 ± 1.0 | n/p | n/a | 1.8 ± 1.4 | 0.01 |
| Ceramides (pmol/mg) | 250 ± 312 | n/p | n/a | 135 ± 174 | 0.08 |
| Sphingomieline (pmol/mg) | 793 ± 810 | n/p | n/a | 511 ± 633 | 0.18 |
| Sphingosine (pmol/mg) | 499 ± 23 | n/p | n/a | 250 ± 174 | 0.01 |
| Sphingosine-1 phosphate (pmol/mg) | 3.5 ± 2.0 | n/p | n/a | 2.0 ± 0.8 | 0.11 |
DED: Dry Eye Disease; n/a: not applicable; n/p: not performed
Table 1: Results of the study.
During the study, a significant reduction was shown for IFN-γ (p=0.01) and sphingosine (p=0.01); a borderline reduction was shown for ceramides (p=0.08).
The prevalences of stages of OSDI score are reported in Table 2. The use of the study treatment achieved a fast reduction of symptoms measured with OSDI questionnaire: at baseline 63% of patients had severe DED symptoms, whereas at both week 2 and 6, normal groups at OSDI were the most prevalent (37% each).
| Baseline | Week 2 | Week 6 | |
|---|---|---|---|
| Normal (OSDI 0-12) | 0 | 0.37 | 0.37 |
| Mild DED (OSDI 13-22) | 0 | 0.21 | 0.16 |
| Mild DED (OSDI 23-32) | 0.37 | 0.1 | 0.26 |
| Severe DED (OSDI 33 or more) | 0.63 | 0.32 | 0.21 |
Table 2: Prevalence of OSDI scores during the study.
Ferning test was just performed on 4 patients and changes were therefore not statistically significant. Yet, the stage was pathological in all patients at the beginning of the study, and just on 25% after 6 weeks of treatment with VisuEvo. The ferning patterns became highly regular with longer and denser ferns (Figures 1 and 2).
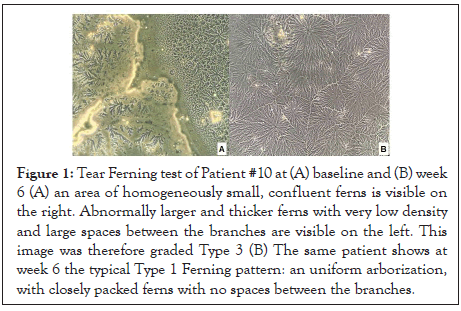
Figure 1: Tear Ferning test of Patient #10 at (A) baseline and (B) week 6 (A) an area of homogeneously small, confluent ferns is visible on the right. Abnormally larger and thicker ferns with very low density and large spaces between the branches are visible on the left. This image was therefore graded Type 3 (B) The same patient shows at week 6 the typical Type 1 Ferning pattern: an uniform arborization, with closely packed ferns with no spaces between the branches.
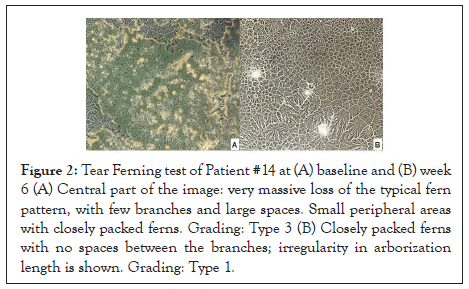
Figure 2: Tear Ferning test of Patient #14 at (A) baseline and (B) week 6 (A) Central part of the image: very massive loss of the typical fern pattern, with few branches and large spaces. Small peripheral areas with closely packed ferns. Grading: Type 3 (B) Closely packed ferns with no spaces between the branches; irregularity in arborization length is shown. Grading: Type 1.
The differences in efficacy in the subgroups of DED were negligible, apart from glaucoma patients showing a lower TBUT recovery (baseline 2.8 ± 1.5 sec, final visit 5.4 ± 2.3 sec, p=0.003) and a lower number of complete blinks throughout the study (p<0.01).
Safety of the device was excellent, as no relevant adverse events were found. Patients’ satisfaction to treatment, using a scale ranging from 0 to 10, was 7 ± 2 at each visit. In the course of the 6 weeks of the study, 35% did not show blurred vision; 65% of patients had sporadic episodes of blurred vision occurring on a median of 2 days (interquartile range, IQR, 1-11 days). The median of cumulative time of blurred vision in the 6 weeks was 46 minutes (IQR 3-77 minutes); the median of annoyance due to blurred vision was 5 (IQR 3-5) on a scale between 0 and 10.
This study highlights the efficacy and tolerability of the new lipid lubricating eye drop VisuEvo in recovering signs and symptoms of DED on a population with evaporative DED and a subgroup of patients with DED associated with mucin impairment. The data presented in this paper are extrapolated from a 3-month study comparing VisuEvo with a standard lipid eye drop [7]. Due to the study design and the statistical analyses performed, the previous study could not fully address the topic of efficacy of tear integration with VisuEvo compared with baseline, which was the focus of this paper.
Lipids have been recently introduced in clinical settings and they are gaining popularity because they seem to have a superior effect than polymers [10-15], at least in evaporative DED [12]. In a recent paper, we also showed how lipids may be useful also in the preventing post-surgical DED [16].
Over the 6-week follow-up of this study TBUT more than doubled (from a mean of 3.0 sec to 6.4 sec) and OSDI score nearly halved (from a mean of 39 to 20). This effect is comparable to the most effective lipid competitors [12,13,17]. Other lipid eye drops obtained lower amelioration of TBUT and OSDI [18,19] or, in case of similar effect, they included patients with less severe DED [10,20].
Even if the findings of this study corroborate the utility of VisuEvo, and more in general lipid-based lubricating eye drops, their mechanisms of action on DED still need to be fully elucidated. Differences in the chemical characteristics of lipid eye drops (composition, size of lipids, formulation as a suspension or solution et cetera) may be critical to determine the clinical success and tolerability. In general lipid-mimetic eye drops increase lipid layer more than polymers [21], but increased lipid thickness does not necessarily imply increased lipid stability [22]. On a group of normal subjects, Markoulli et al. [23] found out that the reduced evaporation lasts about 15 minutes from a single administration of lipid-mimetic eye drop and that this effect is similar to a control with saline solution; yet it is crucial to note that symptoms improved just when subjects received lipids and not saline [23].
It is likely that the clinical success of tear substitutes relies not only on tear volume increase and effective lipid integration, but also on their ability to improve mucin layer (as easily studied by means of Ferning test) and to modulate cellular cross-talking on the ocular surface. DED is associated with tear film hyperosmolarity and increased inflammatory cytokines expression [1]; increased peroxidation has also been recently shown [24]. In our paper, treatment was associated with a statistically significant reduction of tear film osmolarity (from a mean of 328 mOsm/L at baseline, corresponding to moderate DED according to Sullivan et al. [25], to 303-306 under VisuEvo, corresponding to normal values). Also inflammatory indices consistently reduced during the study, and results were statistically significant (despite the low sampling number) for IFN-γ and sphingosine. The role of IFN-γ has been clearly recognized in the pathogenesis of DED, together with IL-1 and IL-6, IL-8, IL-10 and tumor necrosis factor-α [26], whereas the decreased expression of sphingosine still has to be investigated in order to ascertain its biological function in DED. The results of this study may reflect the ability of lipids to reduce evaporation, as well as an immunomodulatory and antinflammatory activity mediated by vitamin D and Omega [3], or the positive trophic effect on mucous tear film layer by Vitamin A [6] or, more likely, a combination of these effects.
Physiologically, focal corneal exposure due to tear evaporation is supposed to drive the blinking reflex by stimulating thermal receptors. In DED, thermal receptor has abnormally high activity, leading to increased blink rate, and in particular to higher frequency of incomplete blinks [27]. Our population had a mean of 7 incomplete blinks per minute at baseline, and 4 at the end of the study, reflecting a better tear film stability and OS homeostasis.
Interestingly, the small subgroup of glaucoma patients showed peculiar patterns: TBUT recovery was lower (final TBUT was 5.4 ± 2.3 sec), as well as the number of complete blinks throughout the study (p<0.01). Also Ferning test (which was abnormal at baseline by definition of inclusion criteria) got better during the study. It is possible that such findings reflect the more severe OS damage occurring in patients chronically treated with preserved medications, leading to OS and eyelid changes and corneal denervation. The chronic use of preserved eye drops is associated with corneal nerve damage [28-30], and subbasal corneal nerves are crucial to preserve the physiological reflexes of the OS (blinking and tearing) and to stimulate epithelial trophism [31]. Previous studies seem to agree on the fact that switching from preserved to non-preserved glaucoma treatments leads to small (about 1 sec) or negligible TBUT improvements but to relevant subjective improvements [32,33]. Better TBUT stabilization is achievable adding non-preserved lubricating eye drops [34], as also confirmed in this paper. Further investigations are necessary to increase our knowledge on the OS changes occurring in glaucoma patients, and the effects of treatments.
In this study, VisuEvo was highly tolerated. We investigated blurred vision in details as this symptom is most commonly complained in DED and when using lipid tear substitutes. A recent survey found out that blurred vision is absent in just 17% of DED patients, whereas it is present at least half of the time in 35% [35]. In our population, patients receiving VisuEvo had complete absence of blurred vision in 35%. Those patients complaining for blurred vision had just sporadic (a mean of less than 2 minutes per day) and poorly disturbing episodes. This is a particularly relevant result considering that our study included patients with severe DED symptoms and patients with longstanding OS damage due to chronic exposure to preservatives.
This report is limited by several method issues, overall due to the fact that it is a re-analysis of just a subgroup of patients from a previous dataset [7]. The number of enrolled patients is small and it was not determined by sample size calculation; we just included all patients receiving VisuEvo in the first period of the cross-over study. Low patient number limits the conclusion which can be drawn on subgroups, for example in patients with mucin defects or those receiving molecular analysis. This study also has a short follow-up: compared with the original dataset, it was halved to 6 weeks. Finally, the study lacks a control group, which would have helped in correctly evaluating the changes occurring during study period, in particular for subjective data such as questionnaire scores, that could be modified by several factors other than treatment efficacy.
Both low- and high-tech devices used to study DED are consistent in showing that VisuEvo has a beneficial effect in stabilizing tear film, reducing subclinical OS inflammation and promoting a stable OS homeostasis. Still, the efficacy of this novel lipid eye drop, and of lipids in general, needs to be further investigated to fully clarify the biochemical interactions occurring on the OS of patients receiving this category of treatment for DED.
Conceptualization, PF; methodology, CQ, PF, AC, MDC; formal analysis, CQ, PF.; investigation, CQ, AC, MDC, PF; writing— original draft preparation, CQ, AC, MDC, PF, LR; writing— review and editing, CQ, AC, MDC, PF, LR;. All authors have read and agreed to the published version of the manuscript.
This study has been supported by private financial funding by Visufarma S.p.A., Rome, Italy. This statement faithfully reflects the authors’ declaration that each of contributing author has not had/has not/will have no financial or proprietary interest in the product studied in the present trial. The study sponsor is also funding the journal’s Open Access Fees.
Paolo Fogagnolo and Luca Rossetti received honoraria for medical meetings and advisory board from Visufarma S.p.A, Rome, Italy.
Citation: Quisisana C, Rossetti L, Caretti A, DeiCas M, Fogagnolo P (2020) The Efficacy of a New lubricating Eyedrop in a Lipid Vehicle for the Treatment of Dry Eye Disease. J Clin Exp Ophthalmol. 11:860. DOI: 10.35248/2155-9570.20.11.860
Received: 02-Oct-2020 Accepted: 16-Oct-2020 Published: 23-Oct-2020 , DOI: 10.35248/2155-9570.20.11.860
Copyright: © 2020 Quisisana C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.