
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2024)Volume 14, Issue 3
Objective: To conduct a systematic review of the efficacy and safety of Shenyankangfu tablets combined with losartan potassium in the treatment of chronic glomerulonephritis.
Method: Through searching CNKI, VIP, Wanfang, Chinese Biomedical Literature Service System, American Clinical Trial Registry, Embase, Web of Science, PubMed, and Cochrane Library databases. The retrieval time was from the establishment of the database to June 1, 2023. All the Randomized Controlled Trials (RCTS) of Shenyankangfu tablets combined with losartan potassium in the treatment of renal chronic glomerulonephritis were collected, and the data of clinical studies meeting the inclusion criteria was extracted. Meta-analysis was performed using Review Manager 5.4 software. The study was registered with PROSPERO, CRD42024499186.
Results: A total of 480 relevant literature was retrieved, and 25 eligible randomized controlled trials were selected, for a total of 2050 participants, including 1025 in the observation group and 1025 in the control group. The results of Meta-Analysis showed: Compared with losartan potassium alone in the treatment of chronic glomerulonephritis, Shenyankangfu tablets combined with losartan potassium can improve the clinical effective rate [RR=1.26, 95% CI (1.20, 1.31) , P<0.001].The reductions of blood urea nitrogen [MD=-0.78, 95% CI (-1.03, -0.53), P<0.001], blood creatinine [MD=-5.06, 95% CI (-8.01, -2.12), P<0.001], 24-hour urine protein quantification [MD=-0.51, 95% CI (-0.63, -0.40), P<0.001], urinary NAG enzyme [MD=-6.77, 95% CI (-12.03,-1.51), P<0.001] and the incidence of adverse reactions [RR=0.59, 95% CI (0.36, 0.95), P<0.001] were better than losartan potassium alone, and the difference was statistically significant.
Conclusion: Shenyankangfu tablets combined with losartan potassium can improve the clinical treatment efficiency of chronic glomerulonephritis, and can effectively reduce the occurrence of blood urea nitrogen, blood creatinine, 24-hour urine protein quantification, urinary NAG enzyme, and adverse reactions.
Chronic Glomerulonephritis (CGN), characterized by clinical manifestations of proteinuria, hematuria, edema, and other symptoms, is common in young and middle-aged males [1], characterized by insipidity of onset, prolonged and difficult to cure, and progressive aggravation, often accompanied by renal function decline. This progresses to chronic kidney failure and eventually uremia [2]. At present, RAS blockers are mainly used for the clinical treatment of chronic glomerulonephritis, and losartan potassium tablets are commonly used as representative drugs of RAS blockers, which can effectively prevent angiotensin receptors from mediating physiological reactions, and reduce proteinuria and the filtration rate of protein in the glomerulus, thereby improving renal function and delaying the development of chronic glomerulonephritis [3]. However, studies have shown that the overall efficacy of losartan potassium tablets alone in the treatment of chronic glomerulonephritis is not ideal [4].
Shenyankangfu tablets are a pure Chinese medicine compound, which consists of 13 traditional Chinese medicines, such as Rehmannia, Eucommia, yam, Salvia miltiorrhiza, Alisma, motherwort, American ginseng, ginseng, and Platycodon, etc. It has the effects of invigorating Qi and nourishing Yin, strengthening the spleen and kidney, and clearing residual toxicity [5-6]. Modern pharmacological experiments have proved that Shenyankangfu tablets have the effect of inhibiting inflammatory response, and has significant efficacy in reducing proteinuria and improving renal function [7]. At present, studies have shown that Shenyankangfu tablets combined with losartan potassium tablets in the treatment of chronic glomerulonephritis can significantly reduce proteinuria content and serum creatinine level, but there is a lack of systematic studies. Therefore, meta-analysis was used in this study to systematically evaluate the efficacy and safety of Shenyankangfu tablets combined with losartan potassium tablets in the treatment of chronic glomerulonephritis, providing medical evidence for clinical application.
Inclusion criteria
Study type Randomized Controlled Trial (RCT) in Chinese or English.
Research object: The included subjects were all clinically diagnosed patients with chronic glomerulonephritis, regardless of race, region, age, gender, disease course, etc.
Intervention measures: The control group was given losartan potassium tablets and the observation group was combined with nephritis rehabilitation tablets based on the control group (the dose and course of treatment were not limited). Outcome indicators\ efficacy indicators: Effective rate, Serum creatinine (Scr), 24-hour urinary protein quantity, urea nitrogen; Safety index: Adverse reactions.Document exclusion criteria
Exclusion criteria: 1. Inconsistent with the research direction; 2. Literature with repeated publication or data duplication; 3. Non- RCTS research; 4. Literature with incomplete data of indicators could not be obtained; 5. Literature with incorrect outcome indicators.
Literature search strategy
Based on the Cochrane Handbook criteria and Preferred Reporting Items for System Reviews and Meta-Analyses (PRISMA statement), our meta-analysis was registered with PROSPERO (CRD42024499186). A computer search was conducted on 5 English databases including PubMed, Web of Science, Cochrane Library, Embase, ClinicalTrials.gov, and China National Knowledge Network (CNKI), VIP Journal Database, Wanfang Journal Papers Database (Wanfang), China Biomedical Literature Database (CBM), which were searched until June 1, 2023. The Chinese search terms were: "Nephritis rehabilitation tablet", "losartan potassium tablet", "chronic glomerulonephritis", "chronic nephritis", etc. The English search terms were: "Glomerulonephritis", "glomerulonephritis’s", "kidney scarring", "losartan potassium", "randomized controlled trial", "RCT", etc. Taking PubMed as an example, the English search strategy was shown in Table 1.
| #1 Search | "Glomerulonephritis"[Mesh] |
| #2 Search | (((Glomerulonephritis’s[Title/Abstract]) OR (Kidney Scarring[Title/Abstract])) OR ((Scarring, Kidney[Title/Abstract]))) OR (Bright Disease[Title/Abstract]) |
| #3 Search | "Losartan potassium"[Mesh] |
| #4 Search | (((((((((((((2-Butyl-4-chloro-1-((2'-(1H-etrazol-5-yl) (1,1'-biphenyl)-4-yl)methyl)-1H-imidazole-5-methanol[Title/Abstract]) OR (DuP-753[Title/Abstract])) OR (DuP 753[Title/Abstract])) OR (DuP753[Title/Abstract])) OR (MK-954[Title/Abstract])) OR (MK 954[Title/Abstract])) OR (MK954[Title/Abstract])) OR (Cozaar[Title/Abstract])) OR (Losartan potassium Potassium[Title/Abstract])) OR (Potassium, Losartan potassium[Title/Abstract])) OR (Losartan potassium Monopotassium Salt[Title/Abstract])) OR (Monopotassium Salt, Losartan potassium[Title/Abstract])) OR (Salt, Losartan potassium Monopotassium[Title/Abstract]))))OR (Shenyankangfu tablets[Title/Abstract]))) |
| #5 Search | (randomized controlled trial[Publication Type] OR randomized[Title/Abstract] OR placebo[Title/Abstract]) |
| #6 Search | #1 OR #2 |
| #7 Search | #3 OR #4 |
| #8 Search | #5 AND #6 AND #7 |
Table 1: PubMed english search.
Literature screening and data extraction
The researchers independently screened the literature according to the inclusion and exclusion criteria, used Note Express software to manage the literature, and excluded the literature that did not meet the requirements according to the title, abstract, and content of the literature, and two entry clerks used Excel to extract and organize the relevant data. Data extraction included: 1. Basic information of the included studies: First author, publication year, etc.; 2. Baseline characteristics of the subjects; 3. Intervention measures, treatment courses, etc.; 4. Outcome indicators, etc.
Quality evaluation criteria
The two researchers evaluated the quality of the included literature according to the Cochrane manual of systematic review and assessed the risk of bias in the input articles to determine:
• Whether random sequences were sufficient
• Whether it is hidden by random allocation
• Double-blind participants and implementers
• Blind method is applied to the result evaluation
• Whether the data results are complete
• Selective reporting
• Other biases. According to the above criteria, the methodological quality included in the research is divided into three levels: "High risk", "low risk" and "unclear risk".
Statistical analysis
Meta-analysis was performed using Review Manager 5.4 software. The Hazard Ratio (HR) was used as the effect size for the bitaxonomic variables, and the Mean Difference (MD) or Standardized Mean Difference (SMD) was used for the continuous variables, and the effect size and its 95% confidence interval were calculated. The test was used to test the heterogeneity of the included studies. If P>0.05 and I² ≤ 50%, the heterogeneity was considered to be small, and the fixed-effect model was selected for analysis. If P ≤ 0.05 and I²>50%, it was considered that there was a large heterogeneity, and the random effects model was selected for analysis.
Literature retrieval process
A total of 480 relevant literature was retrieved. Note Express software was used to eliminate the duplicate pieces of literature and the remaining 423 literature, and the remaining 346 pieces of literature including meta, systematic review, and animal tests were excluded. After reading the title and abstract, 35 works of literature were remained, 10 literatures were eliminated after reading the full text, and 25 literature meeting the criteria was finally included [8-32]. The literature screening process was shown in Figure 1, 2.2 basic features of included studies.
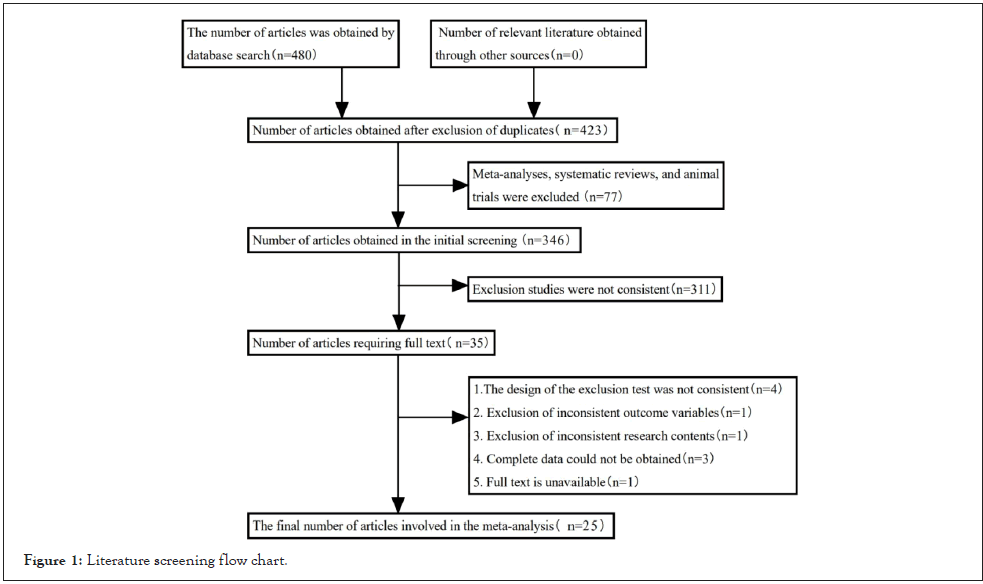
Figure 1: Literature screening flow chart.
Finally, 25 RCTs were included, with a total of 2050 subjects, including 1025 subjects in the observation group and 1025 subjects in the control group. The included studies indicated that baseline information such as age and sex of patients was comparable; Twenty-two studies reported the effective rate of drug treatment [8-20,22-28,30-31], 18 studies reported blood urea nitrogen [8-13,15-17,19-20,22,24-28,30-31], and 21 studies reported blood creatinine [8-10,12-13,15-20,22,24-32]. Twenty-two studies reported 24 h urinary protein quantification [8-10,12-23,25-30,32-33], six studies reported urinary NAG enzyme [9,12,20,26,28,31], and seven studies reported the occurrence of adverse reactions [8,10-12,26,31]. The basic characteristics of the included pieces of literature are shown in Table 2.
|
Study | Number(n)/Age(years) | Treatments | Treatment course | Outcome index | ||
|---|---|---|---|---|---|---|---|
|
(years) | Study group | Control group | Study group | Control group | ||
|
Zhou [8] | 30/44.22 ± 5.32 | 30/43.85 ± 5.22 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 12 weeks | ①②③④⑥ |
|
Liu [9] | 30/44.15 ± 5.37 | 30/45.04 ± 5.45 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 3 months | ①②③④⑤ |
|
|||||||
|
Ru [10] | 45/47.58 ± 11.42 | 45/48.49 ± 11.69 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 8 weeks | ①②③④⑥ |
|
|||||||
|
Guo [11] | 43/35.01 ± 3.12 | 43/34.67 ± 2.35 | SYKF tablet (3.84 g, tid )+Losartan potassium tablet(50 mg, qd) | Losartan potassium tablet (50mg, qd) | 3 months | ①②⑥ |
|
|||||||
|
Chen [12] | 40/41.35 ± 3.65 | 40/41.50 ± 3.40 | SYKF tablet (2.4g, tid )+Losartan potassium tablet (50mg, qd) | Losartan potassium tablet (50mg, qd) | 3 months | ①②③④⑤⑥ |
|
|||||||
|
Yang [13] | 30/53.68 ± 2.51 | 30/53.70 ± 2.47 | SYKF tablet (2.4g, tid )+Losartan potassium tablet (50mg, qd) | Losartan potassium tablet (50mg, qd) | 48 days | ①②③④ |
|
|||||||
|
Luy [14] | 44/50.5 ± 5.3 | 44/51.0 ± 5.5 | SYKF tablet (2.4g, tid )+Losartan potassium tablet (50mg, qd) | Losartan potassium tablet (50mg, qd) | 2 months | ①④ |
|
|||||||
|
Wan [15] | 50/54.88 ± 2.21 | 50/54.21 ± 2.67 | SYKF tablet (2.4g, tid )+Losartan potassium tablet(50mg, qd) | Losartan potassium tablet (50mg, qd) | 12 weeks | ①②③④ |
|
|||||||
|
Zhou [16] | 23/62.48 ± 3.62 | 23/62.51 ± 3.75 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50mg, qd) | 2 months | ①②③④ |
|
|||||||
|
Dong [17] | 15/32.11 ± 2.51 | 15/31.89 ± 2.72 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (20 mg, qd) | Losartan potassium tablet (20mg, qd) | 1 month | ①②③④ |
|
|||||||
|
Li [18] | 50/48.11 ± 2.22 | 50/48.25 ±2.41 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50mg, qd) | 2 months | ①③④ |
|
|||||||
|
Su [19] | 60/34.3 ± 3.2 | 60/35.6 ±4.1 | SYKF tablet (0.6 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 3 months | ①②③④ |
|
|||||||
|
Zhu [20] | 49/54.63 ± 2.72 | 49/53.25 ± 2.38 | SYKF tablet (0.05 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 2 months | ①②③④⑤ |
|
|||||||
|
Guo [21] | 63/45.31 ± 8.24 | 63/46.54 ± 9.51 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 3 months | ④ |
|
|||||||
|
Qiu [22] | 30/52 ± 9.39 | 30/52 ± 8.95 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 3 months | ①②③④ |
|
|||||||
|
Su [23] | 48/43.6 ± 8.6 | 48/43.5 ± 8.5 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 3 months | ①⑥ |
|
|||||||
|
Yan [24] | 30/52 ± 3 | 30/52 ± 3 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 2 months | ①②③④ |
|
|||||||
|
Li [25] | 34/35.01 ± 4.56 | 34/34.78 ± 5.30 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 2 months | ①②③④ |
|
|||||||
|
Qiu [26] | 60/42.9+2.7 | 60/43.4+3.4 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 2 months | ①②③④⑤⑥ |
|
|||||||
|
Sun [27] | 48/52.6 ± 3.8 | 48/51.7 ± 3.3 | SYKF tablet (2.4 g, tid) Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 3 months | ①②③④ |
|
|||||||
|
Zhao [28] | 49/42 ± 6 | 49/42 ± 5 | SYKF tablet (2.4 g, tid )+Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 3 months | ①②③④⑤ |
|
|||||||
|
Wu [29] | 39/45.4 ± 2.3 | 39/46.4 ± 2.5 | SYKF tablet (2.4g, tid ) + Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 12 weeks | ③④ |
|
|||||||
|
Wu [30] | 50/54.4 ± 3.2 | 50/55.4 ± 3.3 | SYKF tablet (0.6g, tid ) + Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 3 months | ②③ |
|
|||||||
|
Zheng [31] | 41/35.8 ± 4.0 | 41/36.4 ± 3.9 | SYKF tablet (2.4g, tid ) + Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 3 months | ①②③④⑤⑥ |
|
|||||||
|
Guo [32] | 24/— | 24/— | SYKF tablet (2.4g, tid ) + Losartan potassium tablet (50 mg, qd) | Losartan potassium tablet (50 mg, qd) | 6 months | ①③④ |
Note: SYKF tablet: Shenyankangfu tablet; —: Not mentioned; ① Effective rate of treatment; ② Blood urea nitrogen; ③ Serum creatinine; ④ 24-hour urinary protein quantification; ⑤ Urinary NAG enzyme; ⑥ The incidence of adverse reactions.
Table 2: Basic characteristics of the literatures included in the study.
Assessment of bias risk of included studies
Cochrane bias risk assessment tool was used to evaluate the quality of the included 25 studies, and the results were as followed: Nine studies [8-10,20,23-25,30-31] used a random number table method and defined it "low risk". Two studies [12,29] proposed randomization according to the order of admission and were defined as "high risk". Fourteen studies [11,13-15,16-19,21-22,26-29] mentioned only the word "random" and were defined as "unclear risk". All studies which did not describe hidden allocations were defined as "unclear risk". The use of blinding was not mentioned and was defined as "unclear risk"; comparing the baseline data of each study all the data was complete, and this no selective report was defined as "low risk". Due to the lack of information provided by the included literature, it was not possible to determine whether there were other biases, which was defined as "unclear risk". As shown in Figures 2 and 3.
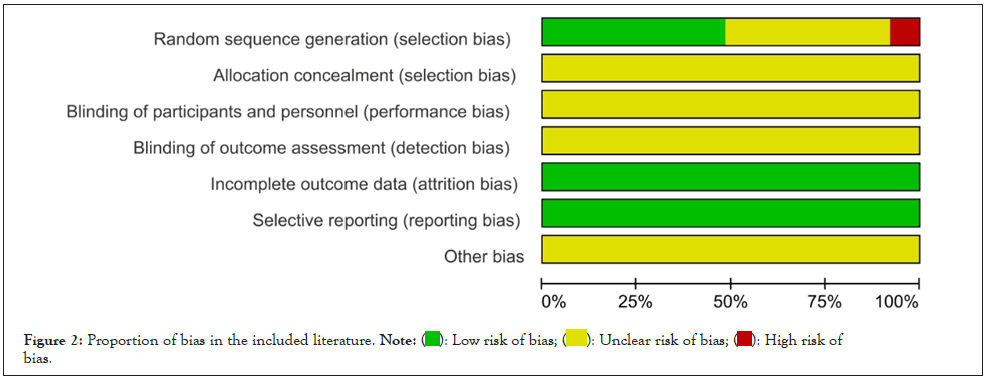
Figure 2: Proportion of bias in the included literature. Note: bias.
bias.
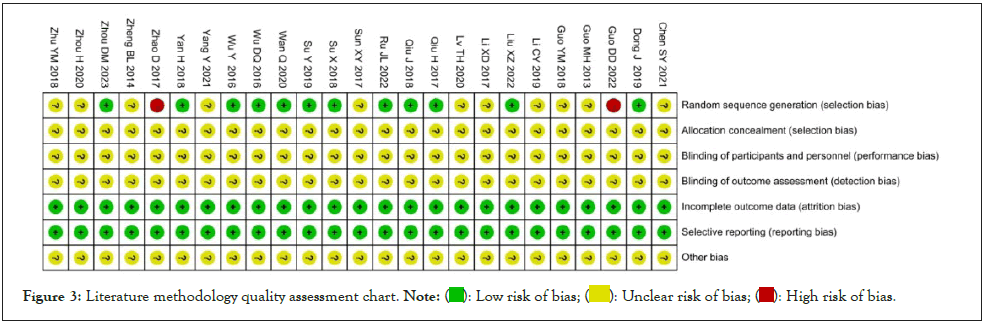
Figure 3: Literature methodology quality assessment chart. Note:  bias.
bias.
Meta-analysis
Effective rate of drug treatment: Twenty-two studies reported the efficacy of drug treatment [8-21,23-29,32,33]. The heterogeneity test showed that the heterogeneity among the kinds of literature selected in this study was not statistically significant (I²=0%, p=0.87). The fixed effect model was used for meta-analysis, and the results showed that: The effective rate of Shenyankangfu tablets combined with losartan potassium tablets in the treatment of chronic glomerulonephritis was better than that of losartan potassium tablets alone [RR=1.26, 95% CI (1.20,1.31), P<0.001], and the difference was statistically significant as shown in Figure 4.
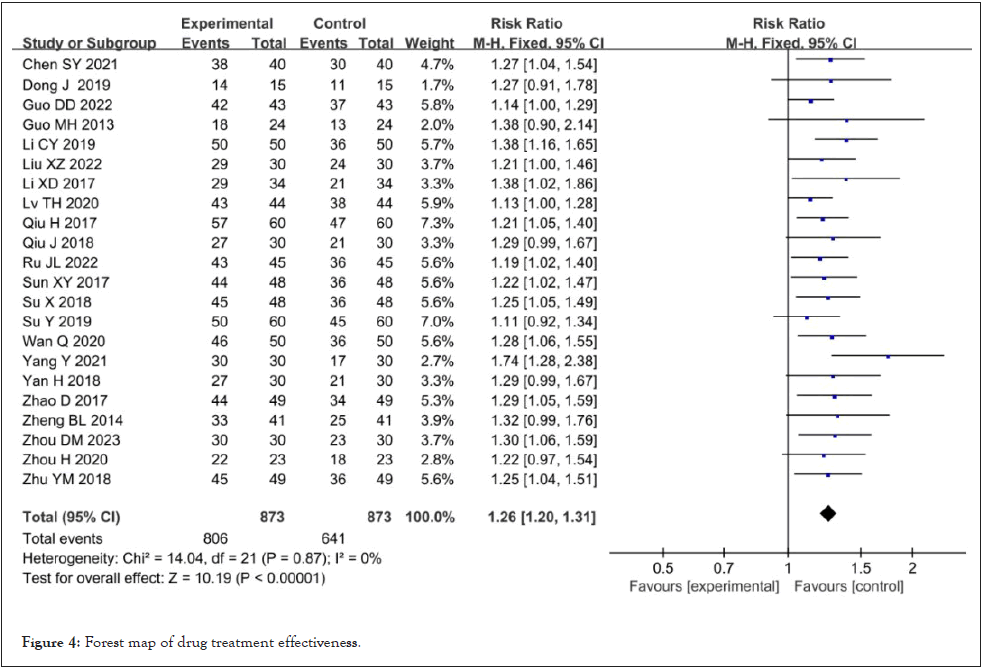
Figure 4: Forest map of drug treatment effectiveness.
Blood urea nitrogen: Blood urea nitrogen was reported in 18 studies [8-13,15-18,20-21,23,25-29,31-32], and there was high heterogeneity after heterogeneity test (I²=91%, P<0.001). A random effect model was used for meta-analysis, and the results showed that Shenyankangfu tablets combined with losartan potassium tablets were better than losartan potassium tablets alone in reducing blood urea nitrogen [MD=-0.78, 95% CI (-1.03, -0.53), P<0.001], and the difference was statistically significant as see Figure 5.
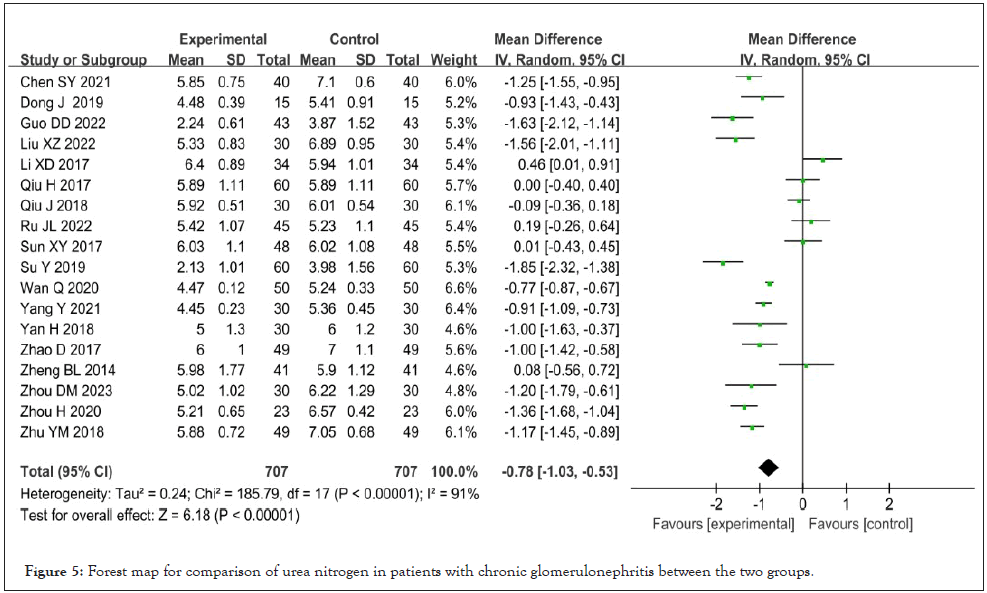
Figure 5: Forest map for comparison of urea nitrogen in patients with chronic glomerulonephritis between the two groups.
Serum creatinine: Serum creatinine was reported in 21 studies [8-10,12-13,15-20,22,24-32]. After the heterogeneity test, there was high heterogeneity (I²=96%, P<0.001). The random effect model was used for meta-analysis, and the results showed that Shenyankangfu tablets combined with losartan potassium tablets in the treatment of chronic glomerulonephritis were better than losartan potassium tablets alone in reducing serum creatinine [MD=-5.06, 95% CI (-8.01,-2.12), P<0.001], and the difference was statistically significant as shown in Figure 6.
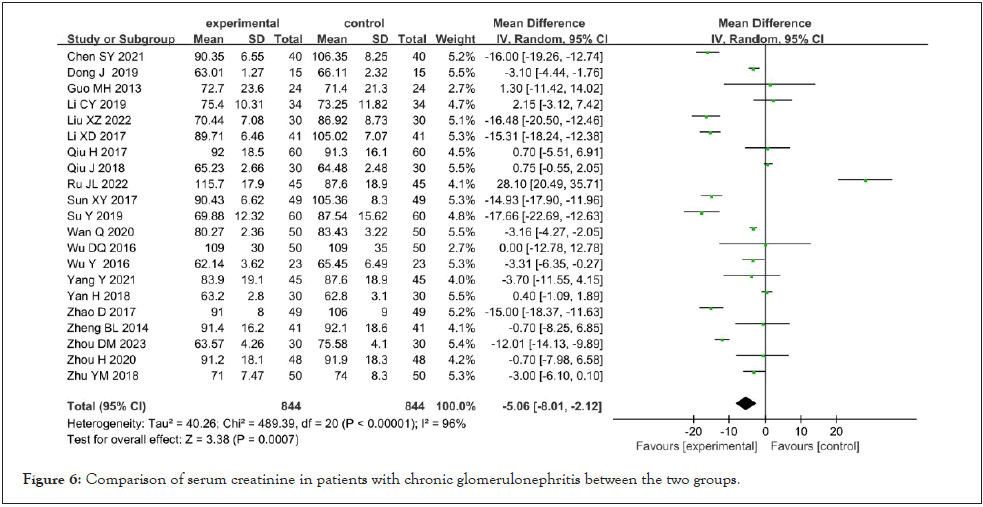
Figure 6: Comparison of serum creatinine in patients with chronic glomerulonephritis between the two groups.
24-hour urinary protein quantification: Twenty-four-hour urinary protein quantification was reported in 22 studies [8-10,12-23,25-30,32-33], which showed high heterogeneity (I²=92%, p<0.001) after heterogeneity test. The random effect model was used for meta-analysis, and the results showed that Shenyankangfu tablets combined with losartan potassium tablets in the treatment of chronic glomerulonephritis were better than losartan potassium tablets alone in reducing 24-hour urinary protein [MD=-0.51, 95% CI (-0.63, -0.40), P<0.001], and the difference was statistically significant as shown in Figure 7.
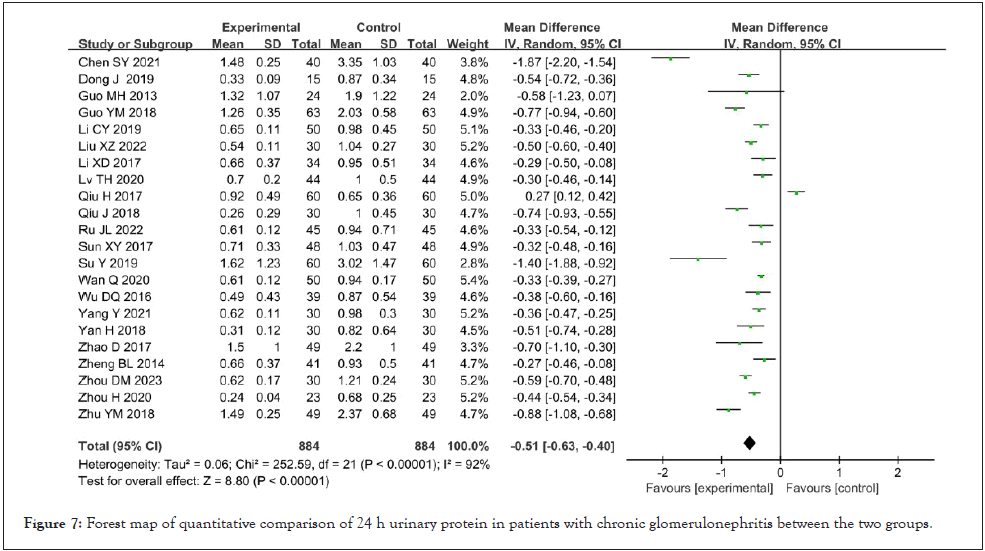
Figure 7: Forest map of quantitative comparison of 24 h urinary protein in patients with chronic glomerulonephritis between the two groups.
Urinary NAG enzyme: Urinary NAG enzymes were reported in 6 studies [9,12,20,26,28,31], which showed high heterogeneity after heterogeneity test (I²=94%, p<0.001). The results showed that Shenyankangfu tablets combined with losartan potassium tablets in the treatment of chronic glomerulonephritis were better than losartan potassium tablets alone in reducing urinary NAG [MD=- 6.77, 95% CI (-12.03, -1.51), p<0.001]. As shown in Figure 8.
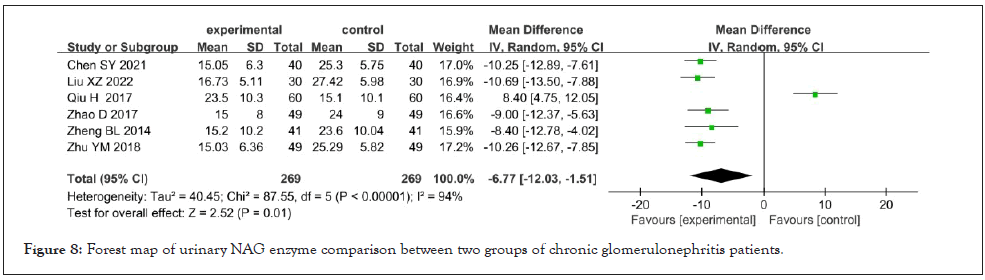
Figure 8: Forest map of urinary NAG enzyme comparison between two groups of chronic glomerulonephritis patients.
Incidence of adverse reactions: Seven studies reported the occurrence of adverse reactions [8-10,12,16,26,31]. After the heterogeneity test, it suggested that the heterogeneity among the kinds of literature selected in this study was not statistically significant (I²=0%, p=0.49). The fixed effect model was used for meta-analysis. The results showed that Shenyankangfu tablets combined with losartan potassium tablets in the treatment of chronic glomerulonephritis had a lower incidence of adverse reactions than losartan potassium tablets alone, [RR=0.59, 95% CI (0.36, 0.95), p<0.001], and the difference was statistically significant. As shown in Figure 9.
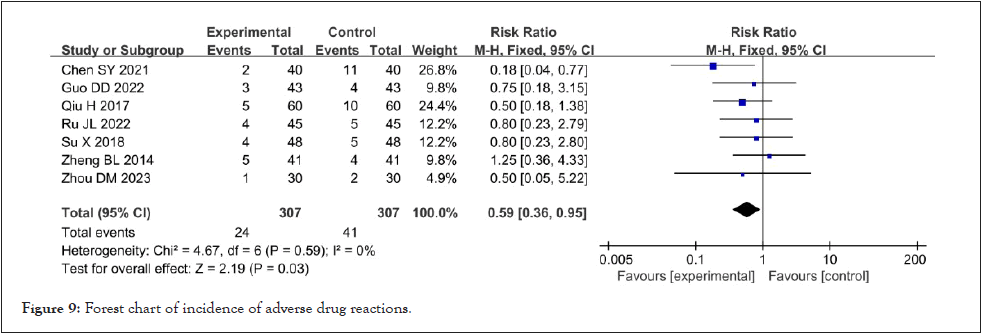
Figure 9: Forest chart of incidence of adverse drug reactions.
Publication bias test: The funnel plot was drawn to investigate whether there was a publication bias in this study, and the effective rate of drug treatment was used to detect the publication bias. The results showed that the funnel plot was symmetrical, indicating that the possibility of publication bias was low, and that the conclusion of this study was reliable as shown in Figure 10.
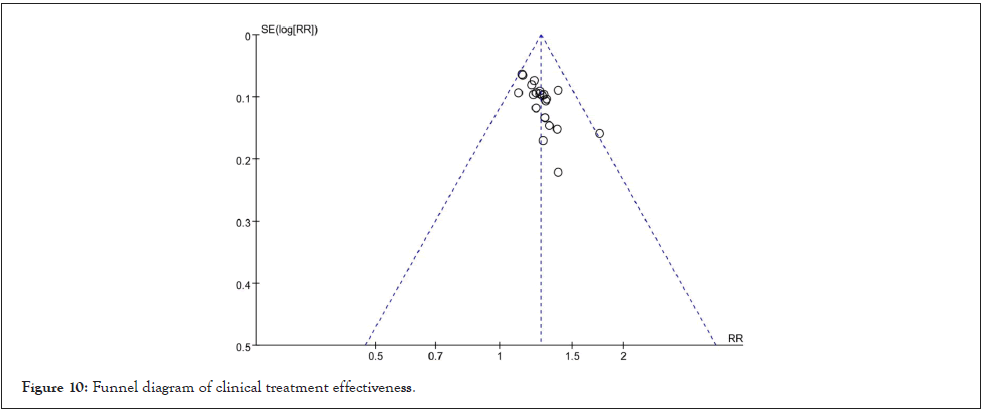
Figure 10: Funnel diagram of clinical treatment effectiveness.
Traditional Chinese medicine believes that chronic glomerulonephritis belongs to the categories of "edema" and "deficiency". The pathogenesis of chronic glomerulonephritis is based on a deficiency of kidney qi and a deficiency of the spleen and kidney. Spleen and kidney deficiency is the pathological basis of chronic glomerulonephritis. Therefore, the main treatment principles should be invigorating the spleen and qi, warming the kidney and tonifying Yang, promoting blood circulation, and removing blood stasis [6]. Shenyankangfu tablets are composed of 13 Chinese herbs, such as raw Rehmannia, yam, Salvia miltiorrhiza, Rhizoma alismatis, motherwort, and white maogen. The combination of all kinds of medicines can mainly tonify deficiency and strengthen the body without dryness, and as well as clear li and dispel pathogens without damaging the body. It has the effect of invigorating qi and nourishing Yin, invigorating the spleen and kidney, and clearing residual toxins. Modern pharmacological experiments have proved that Shenyankangfu tablets have a significant effect on reducing proteinuria and improving renal function [7].
Effectiveness of clinical treatment
The results of the meta-analysis showed that Shenyankangfu tablets combined with losartan potassium tablets were superior to losartan potassium tablets in terms of effective rate, blood urea nitrogen, serum creatinine, 24-hour urinary protein quantification, urinary NAG enzyme, and adverse reactions, and the difference was statistically significant. According to the results of the meta- analysis, Shenyankangfu tablets combined with losartan potassium tablets have certain advantages over losartan potassium tablets alone in the treatment of chronic glomerulonephritis.
Safety of clinical treatment
Seven studies reported the occurrence of adverse reactions [8-10,12,16,26,31], with 24 cases in the observation group and 41 cases in the control group. Adverse reactions included nausea, dizziness, tinnitus, vomiting, mild hyperkalemia, abdominal discomfort, cough, rash, fatigue, etc. However, more than 50% of the included studies did not report the adverse reactions or related treatment measures. More attention should be paid to drug safety, and more attention should be paid to the observation and reporting of safety indicators. The results are shown in Table 3.
| Study | Adverse reaction/case | |
|---|---|---|
| (years) | Study group | Control group |
| Zhou [8] | 1 case of dizziness | There was 1 case of dizziness and 1 case of fatigue |
| Ru [10] | There were 2 cases of dizziness, 1 case of nausea and 1 case of vomiting | There were 2 cases of dizziness, 2 cases of vomiting, and 1 case of rash |
| Guo [11] | Headache occurred in 2 cases and abdominal discomfort in 1 case | There were 1 case of fatigue, 2 cases of headache, and 1 case of abdominal discomfort |
| Chen [12] | There was 1 case of dizziness and 1 case of tinnitus | Dizziness occurred in 6 cases and tinnitus in 5 cases |
| Su [23] | Mild hyperkalemia occurred in 2 cases and dizziness in 2 cases | Mild hyperkalemia occurred in 3 cases and dizziness in 2 cases |
| Qiu [26] | There were 3 cases of dizziness and 2 cases of tinnitus | There were 5 cases of dizziness and 5 cases of tinnitus |
| Zheng [31] | There were 2 cases of cough, 2 cases of mild hyperkalemia, and 1 case of dizziness | Cough and mild hyperkalemia were observed in 2 patients |
Table 3: Occurrence of adverse drug reactions.
Limitations of the study
The deficiencies of this study was as follows:
• None of the RCTs included mentioned blinding and allocation concealment, which resulted in low quality of inclusion and may affect the reliability of the results.
• The number of studies on some outcome indicators was small, and the literature on some indicators such as urinary NAG enzyme was few, and the research results may have certain bias.
• There were differences in the treatment regimens including the dosage of drugs and the course of treatment.
• The sample size of the included studies was small, and there was a lack of multi-center and large-sample studies.
• Most studies did not report adverse reactions, and only 7 studies reported the occurrence of adverse reactions. In conclusion, the results of this study remain cautious, and the results need to be supported by more large-sample, long-term follow-up, and double-blind clinical trials.
This study conducted a meta-analysis of the combination of Shenyankangfu tablets and losartan potassium tablets in the treatment of chronic glomerulonephritis. The combination of Shenyankangfu tablets and losartan potassium tablets have a certain clinical efficacy in treating chronic glomerulonephritis, and the drug safety is good. It provides a new treatment plan for the treatment of chronic glomerulonephritis and can guide clinical treatment.
This study was funded by the National Key Basic Research and Development Program of China (973 Plan) (Grant number 2011CB505406), and the National key research and development program of China [Grant number 2017YFC1703305].
The data sets were analyzed during the present study are available from the corresponding author on reasonable request.
Yang Y, and Jin J conceived and designed this study. Yang Y, and Jin J completed literature retrieval and screening together. Yang Y, and Zhao T analyzed the data and wrote the first draft. Gao Y, Zhu D, Zhang H, Xue Y completed the revision and improvement of the manuscript together. Wang H, Zhao T provided methodological guidance for this study. All authors read and agreed to the final version of the manuscript.
For the acquisition of the data and materials related to this article, please contact the corresponding author Zhao Tieniu (zhaotieniu@163.com).
No competing financial interests exist.
[Crossref] [Google Scholar] [PubMed]
Citation: Yang Y, Jin J, Gao Y, Zhu D, Zhang H, Xue Y, et al. (2024) The Efficacy of Shenyankangfu Tablets Combined With Losartan Potassium for Chronic Glomerulonephritis: A Systematic Review and Meta-Analysis. J Clin Toxicol. 14:566.
Received: 16-Apr-2024, Manuscript No. JCT-24-30805; Editor assigned: 19-Apr-2024, Pre QC No. JCT-24-30805 (PQ); Reviewed: 03-May-2024, QC No. JCT-24-30805; Revised: 10-May-2024, Manuscript No. JCT-24-30805 (R); Published: 17-May-2024 , DOI: 10.35841/2161-1017.24.14.566
Copyright: © 2024 Yang Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.