Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2020)Volume 9, Issue 6
In the present study, the hexane, ethyl acetate and methanol crude extracts of Smilax balbisiana rhizomes were screened for their hypoglycaemic activity using the Oral Glucose Tolerance Test (OGTT) at 300 mg/kg Body Weight (BW). All three crude extracts showed significant post-prandial hypoglycaemic activity when compared with their respective controls (p≤0.05). However, the hexane extract reduced the blood glucose concentration at the glycaemic peak but not as effective as glibenclamide (positive control). The 2, 2-diphenyl-1-picylhydrazyl (DPPH) free radical scavenging assay was used to determine the antioxidant activity where the ethyl acetate (IC50=32.48 μg/mL) and methanol (IC50=58.36 μg/mL) extracts produced moderate inhibition of DPPH radicals when compared with ascorbic acid (IC50=15.88 μg/mL). Several classes of phytochemicals were found within the extracts that may have contributed to the activities observed. This study establishes that the rhizome of Smilax balbisiana contains compounds that contribute to its hypoglycaemic and antioxidant activities and may be beneficial for the management of diabetes.
Smilax balbisiana; Chainy Root rhizomes; Hypoglycaemic activity; Phytochemicals; Antioxidant activity
2, 2-Diphenyl-1-Picrylhydrazyl (DPPH); Diabetes Mellitus (DM); Dimethyl Sulfoxide (DMSO); International Diabetes Federation (IDF); Oral Glucose Tolerance Test (OGTT); phosphatidylinositol 3-kinase/ Protein Kinase B (PI3 K/Akt); Protein of Tyrosine Phosphatase 1B (PTP1B); Sprague-Dawley (S-D); Reactive Oxygen Species (ROS); Standard Error of the Mean (SEM); The University of the West Indies (UWI).
Diabetes Mellitus (DM) is a metabolic disorder that stems from the dysregulation of carbohydrate, protein, and lipid metabolism. It is characterized by an increased blood glucose level (hyperglycaemia) due to the inefficiency or deficiency of insulin [1]. Prolonged hyperglycaemia has been established as the primary cause of diabetesrelated complications and the formation of reactive oxygen species (ROS) [2,3]. At high concentrations, ROS can lead to oxidativestress- mediated organ damage and DNA damage [2]. They can also contribute to the progression of cancer, diabetes, cardiovascular disease, Alzheimer’s, and other degenerative diseases [4].
Presently, diabetes remains in the top five causes of death in the Caribbean. According to the International Diabetes Federation (IDF), diabetes affects 9% of the global adult population, most of whom reside in low to middle-income countries. In Jamaica, 11.7% of the adult population (20-79 years) is affected by type 2 diabetes, and up to 2017, DM was the primary cause of death and disability-affected-years [5,6]. The current management of type 2 diabetes includes drug therapy that is aimed at reducing hyperglycaemia. However, the medicines available have reduced user compliance rates, typically due to their cost and undesirable side-effects [7]. The use of alternative therapy, primarily plant-based remedies (folk/traditional medicine), continues to provide primary health care in developing countries [8]. The increased use of folk medicine versus conventional medicine is particularly evident in rural populations where the practice of folk medicine is frequent [8]. Plants are an established source of bioactive compounds (phytochemicals) that can provide a host of therapeutic benefits as antioxidants, antidiabetic and anti-microbial agents [9]. The use of various phytochemicals continues to influence pharmaceutical drug development due to their contribution to human health and reduced toxicity. Common phytochemicals found to possess therapeutic effects include alkaloids, terpenoids and polyphenols, which have formed the basis for several therapeutic agents used worldwide [10].
Smilax balbisiana (Chainy Root) is a member of the Smilax genus, which is a diverse group of shrubs belonging to the Liliaceae family. It is endemic to Jamaica and is popularly used in “Roots” tonic as an aphrodisiac to treat impotence, increase strength, and purify the blood [11,12]. The rhizomes of this plant are described as “thick, elongated, and tuberous.” They are the core parts used for ethnomedical purposes, as noted by herbalists in the Maroon village of Accompong, Jamaica [12]. Other traditional uses include the treatment of anaemia, syphilis, diabetes, and hypertension [13]. Several species from the Smilax genus are popularly used in Traditional Chinese medicine for the treatment of similar ailments, including syphilis, rheumatoid arthritis, inflammatory diseases, and skin diseases [14]. In other areas, Smilax species have been reported to possess antidiabetic, hypotensive, anti-inflammatory, and other biological properties [15-19].
To the best of our knowledge, no published data have been found regarding the phytochemical constituents or biological activities of S. balbisiana. With this framework, the present study investigated the use of this plant for its hypoglycaemic activity in Sprague- Dawley rats.
Plant material and extract preparation
The University of the West Indies, Mona Campus Research Ethics Committee approved this study in 2015 (Reference No. AN, 8, 14/15). Fresh rhizomes of Smilax balbisiana (Chainy Root) were collected during March and December 2016 from Bethsalem, St. Elizabeth, Jamaica. A specimen was authenticated by Dr Ina Vandebroek (from the New York Botanical Garden, New York, USA) and Mr Patrick Lewis at The University of the West Indies (UWI), Mona Herbarium. A deposit was made and assigned the Voucher number 3633.
Fresh samples of Chainy Root rhizomes were washed to remove extraneous materials and air-dried for five days (shaded from direct sunlight; 30°C). The rhizomes (570 g) were milled to a fine powder, weighed and sequentially extracted with hexane, ethyl acetate and later, methanol. Solvent extraction involved saturating the powder with fresh hexane for two separate eight (8) hour periods and one twenty-four-hour (24) period. The filtrates were collected and concentrated at 60°C using rotary evaporation to obtain the crude hexane extract (1.3 g, 0.23% yield). The procedure was repeated using ethyl acetate (5.3 g; 0.93 % yield) then later, methanol (47.4 g, 8.32% yield). The extracts were stored at -20°C for further studies.
Phytochemical Screening of S. balbisiana crude extracts
The crude extracts of Smilax balbisiana rhizomes were subjected to phytochemical screening using standard methods [20,21]. Dragendorff’s and Hager’s reagents were used to detect the presence of alkaloids while the stain test was used to identify fixed oils. The lead acetate test and alkaline reagent test were used to detect flavonoids. Liebermann-Burchard test and Salkowski’s test were used to detect phytosterols. Benedict’s and Fehling’s reagents were used for reducing sugars. Foam and Froth tests were used to identify saponins and ferric chloride solution for the detection of tannins.
Hypoglycaemic activity (Acute experimental model)
Animals: Healthy Sprague-Dawley (S-D) rats (4-6 weeks old; 150- 200 g) of equally mixed sexes were obtained from the The UWI, Animal House. The rats were divided into groups of six rats each. Before experimentation, they were weighed then fasted overnight (approximately 12 hours) with free access to water.
Oral glucose tolerance test using S. balbisiana crude extracts
An oral glucose tolerance test (OGTT) was performed using a modified protocol [22]. Following an overnight fast, an incision was made at the tip of the rat’s tail, and a fasting blood glucose sample was measured. Immediately after, the crude extract (200 and 300 mg/kg BW), glibenclamide (positive control; 5 mg/kg BW) or carrier (vehicle; negative control) was administered orally. Blood glucose levels were measured for 1 hour at 30-minute intervals, post-administration. After this, a glucose load of 1.75 g/kg BW was administered orally using a gavage needle. Blood glucose readings were taken at 30-minute intervals for a further 2 1/2 hours.
The ACCU-Chek Active Blood Glucose Monitoring System was used to measure blood glucose concentrations. Corn oil or DMSO was used as the carrier (vehicle).
2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) free radical scavenging assay
A modified 2, 2-diphenyl-1-Picrylhydrazyl (DPPH) assay method [23] was used to assess the antioxidant activity of the ethyl acetate and methanol crude extracts from S. balbisiana rhizome. In brief, a solution of DPPH (0.1 mM) was prepared in methanol. Each crude extract was prepared in methanol at varying concentrations (0.08 to 1000 μg/mL). Each reaction mixture consisted of 2 mL of DPPH, which was added to 2 mL of each extract. The mixture was agitated and incubated in the dark at room temperature for 30 minutes. The decrease in DPPH radicals was measured using a spectrophotometer at a wavelength of 517 nm. The reference standard, ascorbic acid (positive control), was prepared similarly at the concentrations mentioned above and assayed in triplicates along with the extracts. A decreased absorbance reading indicated a higher free radical scavenging capacity. Percentage DPPH inhibition was calculated using the formula:
% DPPH inhibition = [(Acontrol-Asample)/Acontrol] × 100
The results were reported as IC50. The IC50 value represents the halfmaximal inhibitory concentration that resulted in 50% inhibition of DPPH radicals.
Statistical analysis
All data were presented as mean ± standard error of the mean (SEM). Statistical analysis was carried out using a statistical package software (SPSS, Version 22, Chicago, IL, USA) to perform the Student’s t-test and Tukey-Kamer multiple range post-hoc test. Variations between the groups where p≤0.05 was considered significant.
The hypoglycaemic effect of the crude extracts from S. balbisiana rhizomes was studied in Sprague-Dawley (S-D) rats using the OGTT. Oral administration of the hexane and ethyl acetate crude extracts (200 mg/kg BW) did not produce any significant change in blood glucose levels when compared with their respective controls (p>0.05; Figures 1 and 2, respectively). However, oral administration of the methanol extract (200 mg/kg BW) resulted in a significant increase in blood glucose level at the 60-minute interval (5.68 ± 0.24 mmol/L vs 4.78 ± 0.25 mmol/L; p=0.025), as well as the 90-minute and 120-minute intervals (p≤0.05) when compared with the control (Figure 3). This hyperglycaemic effect may have been due to the abundance of reducing sugars, which are concentrated in the polar methanol extract, as indicated in the phytochemical analysis (Table 1).
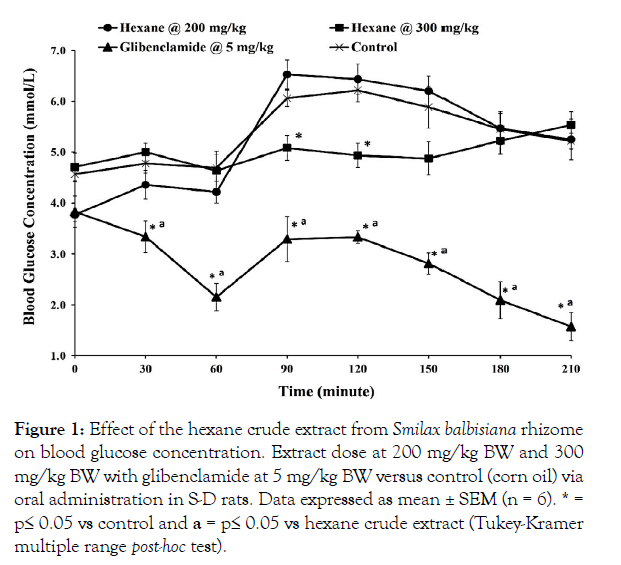
Figure 1: Effect of the hexane crude extract from Smilax balbisiana rhizome on blood glucose concentration. Extract dose at 200 mg/kg BW and 300 mg/kg BW with glibenclamide at 5 mg/kg BW versus control (corn oil) via oral administration in S-D rats. Data expressed as mean ± SEM (n = 6). * = p≤ 0.05 vs control and a = p≤ 0.05 vs hexane crude extract (Tukey-Kramer multiple range post-hoc test).
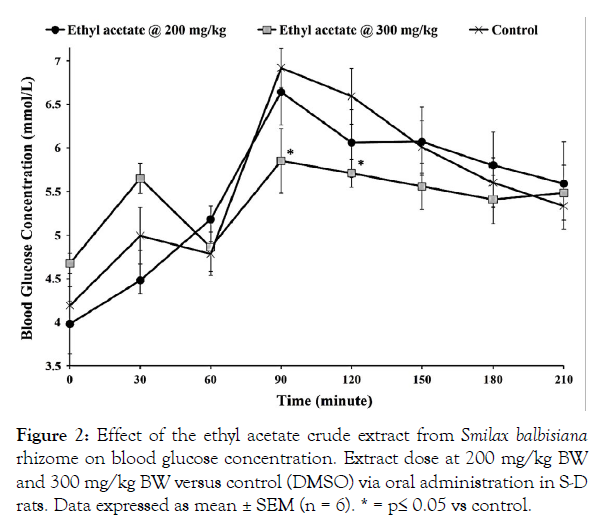
Figure 2: Effect of the ethyl acetate crude extract from Smilax balbisiana rhizome on blood glucose concentration. Extract dose at 200 mg/kg BW and 300 mg/kg BW versus control (DMSO) via oral administration in S-D rats. Data expressed as mean ± SEM (n = 6). * = p≤ 0.05 vs control.
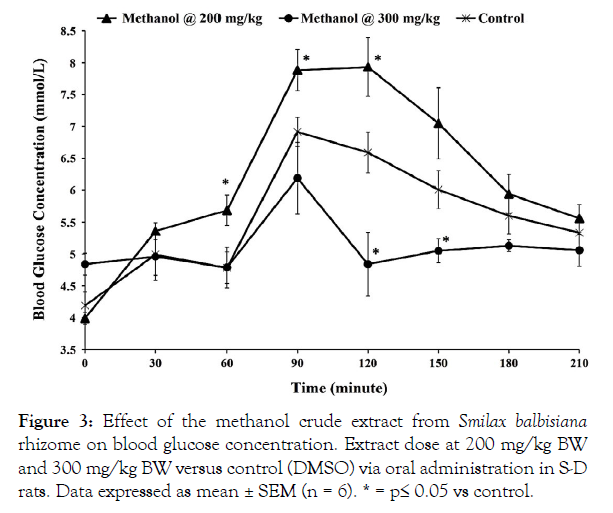
Figure 3: Effect of the methanol crude extract from Smilax balbisiana rhizome on blood glucose concentration. Extract dose at 200 mg/kg BW and 300 mg/kg BW versus control (DMSO) via oral administration in S-D rats. Data expressed as mean ± SEM (n = 6). * = p≤ 0.05 vs control.
| Phytoconstituents | Test (s) | Hexane extract | Ethyl acetate extract | Methanol extract |
|---|---|---|---|---|
| Alkaloids | Dragendorff’s test | ++ | + | - |
| Hager’s test | ++ | + | - | |
| Fixed oils | Stain test | ++ | + | + |
| Flavonoids | Lead acetate test | - | + | + |
| Alkaline reagent test | - | + | + | |
| Phytosterol and terpenoids | Lieberman-Burchard’s test | ++ | + | + |
| Salkowski’s test | ++ | + | + | |
| Reducing sugars Carbohydrates) | Benedict’s test | - | + | ++ |
| Fehling’s test | - | + | ++ | |
| Saponins | Foam test | - | + | ++ |
| Froth test | - | + | ++ | |
| Â Tannins | Ferric chloride test | - | + | + |
Table 1: Phytochemical constituents of Smilax balbisiana rhizome extracts.
In contrast, an increase in the dosage of each extract displayed significant improvement in blood glucose tolerance. The administration of S. balbisiana hexane extract (300 mg/kg BW) was able to produce the greatest hypoglycaemic response, following an oral administration of glucose. A significant reduction was observed at the 90-minute interval (5.08 ± 0.24 mmol/L vs 6.06 ± 0.16 mmol/L; p = 0.007) and the 120-minute interval (4.93 ± 0.24 mmol/L vs 6.22 ± 0.23 mmol/L; p = 0.003) when compared with its control, corn oil (Figure 1). The hexane extract was compared with an oral hypoglycaemic agent, glibenclamide (5 mg/kg BW). Glibenclamide produced significant hypoglycaemia throughout the glucose tolerance test when compared with both the hexane crude extract and the control (p ≤ 0.01; Figure 1). Glibenclamide is known to stimulate insulin release from the pancreatic β-cells, which leads to its hypoglycaemic effect [24]. This hypoglycaemic effect was quite pronounced in the normal healthy rats used in this study. The variation in blood glucose levels observed between the hexane crude extract and glibenclamide may be due to the presence of numerous compounds in the crude extract. myriad of compounds in this crude extract could have masked the potency of the hypoglycaemic compound(s) present.
The ethyl acetate extract (300 mg/kg BW) also reduced the blood glucose level when compared with its control, DMSO. This lowering was observed at the 90-minute interval (5.85 ± 0.37 mmol/L vs 6.92 ± 0.22 mmol/L; p=0.033) and 120-minute interval (5.71 ± 0.16 mmol/L vs 6.59 ± 0.32 mmol/L; p=0.034; Figure 2). In particular, the methanol extract (300 mg/kg BW) was able to counter the hyperglycaemic activity displayed following administration at 200 mg/kg BW. The increased dosage displayed hypoglycaemic responses at the 120-minute interval (4.84 ± 0.50 mmol/L vs 6.59 ± 0.32 mmol/L; p=0.014) and 150-minute interval (5.05 ± 0.19 mmol/L vs 6.01 ± 0.30 mmol/L; p = 0.03) in comparison with its control, dimethyl sulfoxide (DMSO; Figure 3). The increased dosage of the methanol extract would have allowed the active compounds to reach sufficient levels to mitigate the effect of the high sugar content as observed (Figure 3).
As seen in these three crude extracts, it is possible that at a lower dosage, the other compounds present might have nullified the activity of the active compound(s) or the activity of the hypoglycaemic compound(s) might be present in negligible quantity. However, as the concentration increased the active compound(s) was present in sufficient concentration to exert the hypoglycaemic effect observed [25].
Preliminary phytochemical analysis of Smilax balbisiana rhizomes detected an abundance of alkaloids, fixed oils, phytosterols, and terpenoids in the hexane crude extract. A moderate amount of all phytoconstituents tested was present in the ethyl acetate crude extract. The similarity in the amount of phytochemicals detected could have indicated why the hypoglycaemic effect of the ethyl acetate extract was not as notable as that displayed by the hexane extract. In contrast, the methanol extract contained a moderate concentration of flavonoids, fixed oil, tannins, terpenoids, and phytosterols, along with an abundance of reducing sugars and saponins (Table 1). In particular, saponins have been reported to exert hypoglycaemic effect via numerous mechanisms, including the promotion of insulin release from the beta-cell islets, activation of glycogen synthesis, inhibition of gluconeogenesis, inhibition of α-glucosidase, and inhibition of mRNA expression of glycogen phosphorylase and glucose 6-phosphatase [26]. Furthermore, several of these classes of phytochemicals have been linked to the hypoglycaemic and antidiabetic effect of Smilax china L. rhizome [27]. The mechanism by which these phytochemicals produce hypoglycaemia mainly involves an increase in pancreatic glucose uptake, the inhibition of intestinal alpha-glucosidase activity, or the stimulation of insulin secretion [28].
The hexane crude extract contained a high concentration of phytosterols/terpenoids and alkaloids. Phytosterols are known to stimulate insulin release, suppress glucose levels, and increase the absorption of other molecules to improve glucose tolerance [29]. Terpenoids (phytosterols) have also demonstrated antidiabetic activity via activation of AMPK and inhibition of the alphaglucosidase enzyme [30,31]. Alkaloids have been reported to exhibit antidiabetic effects by activation of the phosphatidylinositol 3-kinase/Protein Kinase B (PI3 K/Akt) insulin signalling pathway and suppression of protein of tyrosine phosphatase 1B (PTP1B), as well as via promotion of glycogen synthesis [32]. A previous report highlighted that the biological effects of Smilax species had been attributed to the presence of saponins and phytosterols, which was found most abundant in the hexane extract [33]. The actions of the phytosterols, terpenoids, and alkaloids from the hexane extract are likely to have led to the significant increase in the rate of glucose clearance as observed in the post-prandial region of the OGTT (Figure 1).
In general, an increased dosage of the crude extracts resulted in significant hypoglycaemic responses when compared with their respective controls. These responses indicated that a dose-dependent effect was associated with the hypoglycaemic activity of S. balbisiana rhizome extracts. Crude plant extracts are known to contain an unspecified number of phytochemicals. The concentration of these compounds may influence the action of the bioactive compound(s) present. It was observed that the crude extracts had the potential to reduce blood glucose levels significantly. Therefore, the hypoglycaemic activity could become more effective upon isolation of the bioactive compound(s).
Analysis of free radical scavenging activity of the ethyl acetate and methanol crude extracts indicated a concentration-dependent antioxidant effect. The DPPH scavenging activity (%) of the ethyl acetate and methanol extracts increased from 0.8 μg/mL to 1000 μg/mL. The ethyl acetate extract increased from 26.72 ± 0.333 % to 89.34 ± 0.00% while methanol increased from 26.93 ±.57% to 83.66 ± 0.33%. Ascorbic acid showed the strongest free radical scavenging activity with an increase from 27.99 ± 1.15 % to 94.42 ± 0.33% at the maximum concentration (Table 2). From the IC50 values obtained (Table 2), it was evident that the ethyl acetate extract (IC50=32.48 μg/mL) had a more substantial DPPH radical scavenging effect than the methanol extract (IC50=58.36 μg/mL) and was comparable to the positive control, ascorbic acid (IC50 =15.88 μg/mL). Similar results were reported for S. chinensis L. where the ethyl acetate extract produced a more potent antioxidant effect compared with the methanol extract [34,35]. The antioxidant effect was reported to contribute to the significant hepatoprotective effect of the S. chinensis L [33]. S. larvata ethyl acetate extract also produced moderate antioxidant activity compared to ascorbic acid. It was linked to the presence of various flavonoids, including kaempferol which is found throughout the Smilax genus [35]. The hydrogen donating ability of the ethyl acetate extract can be linked to the presence of ubiquitous phytochemicals, including flavonoids, tannins, and saponins, which have established antioxidant properties [9,36]. These compounds may, therefore, protect against hyperglycaemia-induced oxidative stress that contributes to the development of diabetic complications [37].
| Extract | Free Radical Scavenging Activity (%)a | IC50 (µg/mL) |
|---|---|---|
| Ethyl acetate | 89.34 ± 0.00 | 32.48 |
| Methanol | 83.66 ± 0.33 | 58.36 |
| Ascorbic acid (positive control) | 94.42 ± 0.33 | 15.88 |
Table 2: 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) free radical scavenging effect of various crude extracts on Smilax balbisiana.
Oral administration of the crude extracts of Smilax balbisiana rhizome indicated a dose-dependent hypoglycaemic effect. The hexane extract at 300 mg/kg BW produced a significant glycaemic lowering in Sprague-Dawley rats. The hypoglycaemic effect of the hexane crude extract was attributed to the presence of alkaloids, phytosterols, and terpenoids, which were detected at high concentrations. The antioxidant assay indicated that the ethyl acetate crude extract, which contained moderate concentrations of polyphenols and flavonoids, produced modest free radical scavenging effect against DPPH free radicals. This study provides beneficial insight into the use of Smilax balbisiana (Chainy Root) rhizome in the management of hyperglycaemia as well as its potential antioxidant effect against hyperglycaemic-induced oxidative stress associated with diabetes. The ethnopharmacological properties of S. balbisiana extracts can lead to its integration as an adjunct or alternative therapy in diabetes management.
The authors acknowledge the Office of Graduate Studies and Research, The University of the West Indies, Mona, for their financial support. The authors also thank Mr Patrick Lewis for authenticating the plant.
D. A. Peddie – Performed data collection, analysis, and interpretation of data; wrote the manuscript.
C. S. Bowen-Forbes – Provided critical revision of manuscript and interpretation of data.
R. L. Alexander-Lindo – Conceptualized and designed study; assisted with the preparation of the manuscript and corresponding author.
All authors have contributed to and approved the final manuscript. We declare no conflict of interest in the publication of this manuscript.
Citation: Peddie DA, Bowen-Forbes CS, Alexander-Lindo RL (2020) The Ethnopharmacological Effects of Crude Extracts from Smilax balbisiana (Chainy Root) Rhizomes. Med Aromat Plants (Los Angeles) 9: 364.
Received: 12-Sep-2020 Accepted: 07-Nov-2020 Published: 13-Nov-2020
Copyright: © 2020 Ruby L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.