
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Case Report - (2023)Volume 13, Issue 2
Background and aims: Appendiceal Mucinous Neoplasms (AMNs) are an uncommon malignancy affecting the appendix. The observed incidence is less than 1% among all appendectomy patients, with most cases occurring in middle aged and elderly patients. Although it is commonly referred to as the mucocele of the appendix, this terminology is used to describe a distended, mucus filled appendix. It is an ambiguous term best used to convey an imaging appearance rather than a pathologic entity because appendiceal mucinous lesions' underlying biology and behavior are incredibly variable, ranging from benign to neoplastic. Previously, the differentiation between benign and neoplastic appendiceal mucoceles was challenging; however, ten years ago, the Peritoneal Surface Oncology Group International (PSOGI) resolved this issue by establishing a consensus classification. They classified it into non neoplastic appendiceal mucinous lesions and neoplastic appendiceal mucinous lesions. The first is mainly a simple mucocele, which is attributed to obstruction and distention of the appendix, caused by degenerative epithelial changes without any evidence of neoplasia or hyperplasia.
Mucocele; Appendix; Mucinous; Neoplasm; Tumour; Colorectal
Appendiceal Mucinous Neoplasms (AMNs) are an uncommon malignancy affecting the appendix. The observed incidence is less than 1% among all appendectomy patients, with most cases occurring in middle aged and elderly patients [1]. It is common to misdiagnose this condition, as most cases show no usual clinical signs [2]. Early stage AMNs are generally discovered incidentally upon appendicectomy for suspected appendicitis. The accumulation of mucin in the peritoneum causes abdominal distension in the advanced stage of the diseases. Only a minor proportion of individuals have nonspecific symptoms such as chronic right lower abdominal pain or a mass [3].
Pre-operative diagnosis is essential to avoid tumor rupture during surgery, which can result in iatrogenic implantation and the development of Pseudomyxoma Peritonei (PMP), which affects about 20% of individuals with MANs but considerably fewer people with non-mucinous appendiceal adenocarcinoma [4]. AMNs can be classified as Low grade (LAMN), High grade (HAMN), and mucinous adenocarcinoma [5]. Ultrasound, Computerized Tomography (CT), and Magnetic Resonance Imaging (MRI) is the most common AMN imaging modalities. Moreover, many quantitative prognostic scoring methods have been developed. They are used to determine the best candidates for surgery, including the Peritoneal Cancer Index (PCI), the Peritoneal Surface Disease Severity Score (PSDSS), and the Simplified Pre-operative Assessment for Appendix Tumor (SPAAT) [6-8]. Survival rates in these patients can be dramatically improved with proper therapy. Complete Cytoreductive Surgery (CRS) in combination with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) can result in 5 years survival rates in about two-thirds of these patients [9,10]. We present a case of LAMN that was identified and treated at our institution based on surgical and histological results. The most recent literature was also examined to raise awareness and understanding of this condition.
On the other hand, the neoplastic appendiceal mucinous lesions are further classified into serrated polyps of the appendix, AMNs, and mucinous adenocarcinomas in the appendix. AMNs are further classified into Low grade (LAMN) and High grade (HAMN). It is common to misdiagnose this condition, as most cases show no clinical signs. Early-stage AMNs are generally discovered incidentally upon appendicectomy for suspected appendicitis. The accumulation of mucin in the peritoneum causes abdominal distension in the advanced stage of the disease. This can progress into the development of Pseudomyxoma Peritonei (PMP), known for its high mortality, if not identified promptly and treated definitively. Pre-operative diagnosis is essential to avoid tumor rupture during surgery, which can result in iatrogenic implantation and the development of PMP, which affects about 20% of individuals with AMNs but considerably fewer people with non-mucinous appendiceal adenocarcinoma. Multiple imaging modalities can aid the diagnosis of AMN, such as ultrasound, Computerized Tomography (CT), and Magnetic Resonance Imaging (MRI). Surgical resection is the gold standard treatment for LAMN, considering that it is the only therapeutic option that offers curability. A complete right hemicolectomy is not recommended in patients with a LAMN or HAMN with no evidence of extraappendiceal neoplastic epithelial involvement, where a simple appendectomy suffices. However, there is some controversy around managing cases with microscopically positive margins following the appendectomy of an un-ruptured LAMN. Some recommendations support re-excision by cecectomy or ileocecectomy in these cases, while others advise against a complete right hemicolectomy. Furthermore, a right hemicolectomy appears to provide no substantial benefit over appendectomy alone in patients with cellular or acellular mucin on the serosal surface of the appendix but no distant peritoneal mucinous disease, despite the higher risk of developing recurrence and PMP. This paper aims to provide an overview of the management dilemmas of AMNs through a review of the most recent literature while exemplifying this with a case of an incidental LAMN managed in our institution.
Despite the rare incidence of LAMN, early diagnosis and appropriate surgical management can increase the survival rate and reduce the risk of PMP. Ultrasound, CT, MRI, and colonoscopy are valuable diagnostic modalities of LAMN and its consequences. The gold standard in treating patients with LAMN is surgical resection. However, there is controversy on the extent of resection in such cases. Evidence supporting right hemicolectomy is very limited and against the current international recommendations. Even when histological examination reveals cellular mucin on the serosal surface of the appendix, which adds the risk of recurrence and developing PMP, post-treatment surveillance, which involves cross-sectional imaging and tumor markers, should be adapted to identify the risk of recurrence based on pathology findings and surgical resection completeness. In LAMN, there is currently no agreement on surveillance imaging. Most of the literature recommends annual CT imaging, which may be terminated after 5–10 years if the tumor or mucin has not extended beyond the appendix and when no sign of recurrence has been identified. In completely resected LAMN, specific follow-up or surveillance is unnecessary due to the absence of recurrence risk.
A 75-year-old male was admitted to the hospital with the incidental finding of appendicular mucocele during routine Computed Tomography (CT) Positron Emission Tomography (PET) to stage malignant melanoma of the scalp. At presentation, the patient appeared to be physically well and had no notable symptoms or signs. They had no concerning red flags for colorectal cancer (unexplained weight loss or bleeding) and no history of inflammatory bowel disease. His medical history revealed prior hospital admission in 2021 for acute swelling of the appendix suggestive of acute appendicitis, which was managed conservatively. A few weeks later, he underwent a colonoscopy with no remarkable findings. Upon the incidental identification of the mucocele, the patient was brought forward for a repeat colonoscopy to exclude malignancy or polyps at the appendix orifice. The findings of the colonoscopy were unremarkable, aside from hyperplastic polyps. A consensus from the multidisciplinary meeting advised the patient to undergo appendicectomy, which may be extended to right hemicolectomy. The patient underwent laparoscopic ileocolic resection under general anesthesia. A large appendiceal mucocele was adherent to the terminal ileum with two prominent serosal nodules on the adjacent caecum. This prompted the decision to proceed with an ileocolic resection to ensure clear resection margins without risking spillage. The histological examination revealed LAMN, pT3N0, and 0/15 lymph nodes, with clear resection margins and no mucin on the surface. The patient underwent routine post-operative care and was followed up for two weeks postoperatively. The patient course was uneventful at the end of the follow-up [11].
Investigations
Initially, the patient underwent a colonoscopy to exclude malignancy or polyps at the appendix orifice. The findings of the colonoscopy were unremarkable, aside from hyperplastic polyps. Then, the patient underwent an abdominal CT scan to assess the progression of the swelling, which revealed gross dilatation of the mucocele. A consensus from the multidisciplinary meeting advised the patient to undergo appendicectomy, which may be extended to right hemicolectomy. Preoperatively, the serum Carcinoembryonic Antigen (CEA) level was measured at three ng/dL [12].
Surgical management
The patient underwent laparoscopic ileocolic resection under general anesthesia. A large appendiceal mucocele was adherent to the terminal ileum with two prominent serosal nodules on the adjacent caecum. The caecum was folded superiorly onto ascending colon, with no free mucin. The surgeon observed no intraoperative perforation or spillage. Initially, an attempt at the free dissection of the appendix from the terminal ileum was made; however, the appendix was firmly adherent to the ileum at the mid-body [13]. This prompted the decision to proceed with an ileocolic resection to ensure clear resection margins without risking spillage. The caecum was mobilized lateral to medial, and the terminal ileal mesentery was mobilized up to the duodenum. The dissection was made under the ileocolic pedicle to meet the lateral dissection plane and sweep down the duodenum. The ileocolic pedicle was then skeletonized and ligated low with LigaSure™. Mobilization of the right colon was then completed superiorly to meet the inferior dissection plane. After checking the mobility and hemostasis, the umbilical wound was extended, and an Alexi’s wound retractor was placed. The right colon and terminal ileum were easily exteriorized. Points of division were chosen on the ascending colon and terminal ileum (Figures 1 and 2). The colonic mesentery was ligated, and the marginal vessel was tested, showing good flow. The small bowel mesentery was ligated with LigaSure™. Stapled side to side (functional end to end) ileocolic anastomosis was fashioned with a GIA 80 blue stapler for the longitudinal staple line and a TA90 blue stapler for the transverse staple line [14]. Two areas of minor ooze from the longitudinal staple line were oversewn with 3/0 PDS after the inspection in the lumen following the first stapling. The transverse staple line was buried, and crotch sutures were placed with 3/0 PDS. The hemostasis was checked, and the bowel was returned to the abdomen. An intraperitoneal local anesthetic catheter was inserted in the right upper quadrant. The fascia of the umbilical wound cleared and closed with continuous 0 Maxon. 3/0 Monocryl to skin [15].
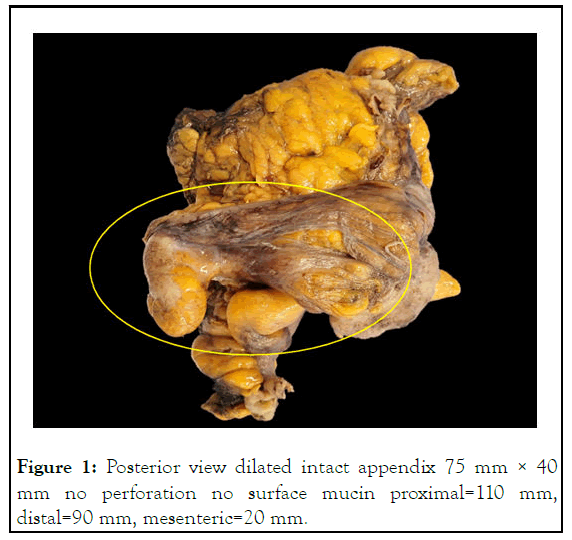
Figure 1: Posterior view dilated intact appendix 75 mm × 40 mm no perforation no surface mucin proximal=110 mm, distal=90 mm, mesenteric=20 mm.
Outcome and follow-up
The histological examination revealed LAMN, pT3N0, and 0/15 lymph nodes, with clear resection margins and no mucin on the surface [16]. There was a normal mismatch repair without BRAF mutation (Figures 2 and3).
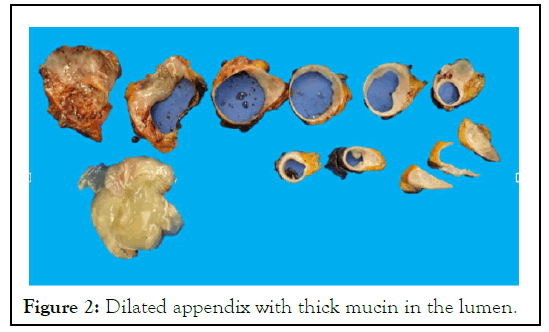
Figure 2: Dilated appendix with thick mucin in the lumen.
The patient underwent routine post-operative care and was followed up for two weeks postoperatively. The patient course was uneventful at the end of the follow-up [17].
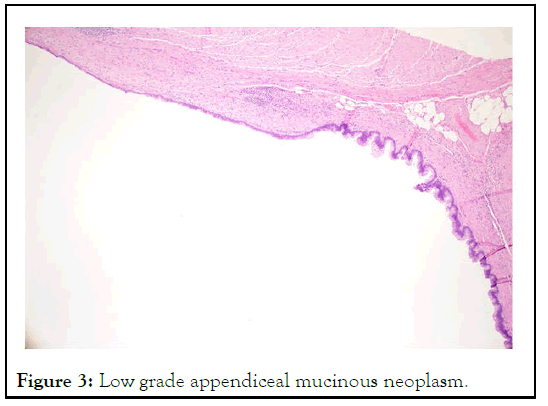
Figure 3: Low grade appendiceal mucinous neoplasm.
Although a mucocele is used to describe a distended, mucusfilled appendix, the term is ambiguous. It is best used to convey an imaging appearance rather than a pathologic entity because appendiceal mucinous lesions' underlying biology and behavior are incredibly variable, ranging from non-neoplastic to neoplastic. Previously, the differentiation between benign and neoplastic appendiceal mucoceles was challenging; however, ten years ago, the Peritoneal Surface Oncology Group International (PSOGI) resolved this issue by establishing a consensus classification. They classified it into non-neoplastic mucinous lesions and neoplastic mucinous lesions. The first is mainly simple mucoceles, which are attributed to obstruction and distention and described as degenerative epithelial changes without any evidence of neoplasia or hyperplasia. The neoplastic appendiceal mucinous lesions are further classified into serrated polyps of the appendix, MANs, and mucinous adenocarcinomas of the appendix (Figure 4) [18].
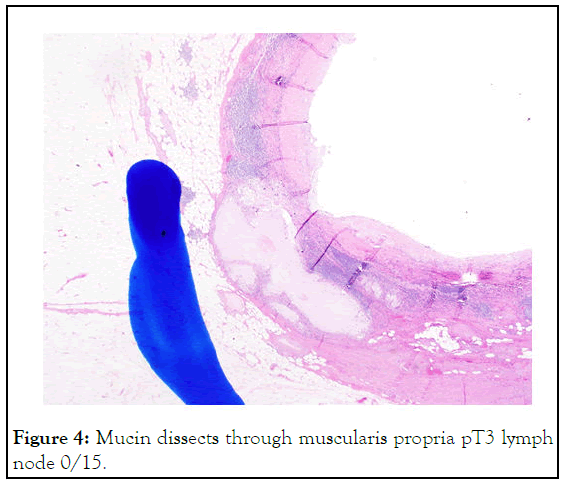
Figure 4: Mucin dissects through muscularis propria pT3 lymph node 0/15.
As previously mentioned, AMNs are classified into two major types: LAMN and HAMN. In this case, we present a patient with LAMN who underwent a complete resection to manage his condition. Surgical resection is the gold standard for LAMN since it is the only therapeutic option with a chance of being curative. A complete right hemicolectomy is not recommended in patients with a LAMN or HAMN with no evidence for extra-appendiceal neoplastic epithelial and is removed by appendectomy. However, this point is controversial; some recommendations support re-excision by cecectomy or ileocecectomy for a microscopically positive margin following appendectomy for an un-ruptured LAMN, while others advise against a complete right colectomy [19].
Patients with cellular or acellular mucin on the serosal surface of the appendix or the mesoappendix but no distant peritoneal mucinous disease are classified as T4a. The risk of severe PMP is often greater in these individuals than in those who do not have perforation. Most research demonstrated that individuals with just acellular mucin deposits have recurrence odds of 3% to 7%, whereas those with cellular mucin have a risk of 33% to 78%.
There is no consensus on how to handle T4a LAMN patients effectively. Despite the increased risk of recurrence, right hemicolectomy appears to provide no substantial benefit over appendectomy alone. Therefore, most institutions typically monitor patients with PMP with routine imaging and tumor markers. CRS and HIPEC are used to treat PMP once it has been discovered. In addition, CRS and HIPEC are utilized in cases with peritoneal mucin (M1a/b disease) [19,20]. Even though LAMN is a benign tumor, the peritoneal spread of mucinous disease puts patients at risk for future recurrences and PMP. Furthermore, CRS and HIPEC should be considered the first line of therapy for such stage IVA patients.
For those with acellular mucin limited to the right lower quadrant, the American society of colon and rectal surgeons recommended appendectomy with periappendiceal peritoneum cytoreduction, but HIPEC without CRS for those with cellular mucin because of a higher risk of peritoneal. M1a/b LAMN is divided into three groups by the Chicago consensus LAMN management pathway: Local acellular mucin, widespread acellular mucin, and cellular mucin. For the latter two groups of patients, CRS and HIPEC are unequivocally indicated. Those with peritoneal diseases localized to the right lower quadrant probably had their disease removed at the time of appendectomy. In that case, subsequent therapeutic options include monitoring and intraperitoneal chemotherapy. Patients with peritoneal disease in the right lower quadrant that is not entirely resected can choose between observation and CRS/ HIPEC.
Post-treatment surveillance, which involves cross-sectional imaging and tumor markers, should be adapted to the risk of recurrence based on pathology and surgical resection completeness. In LAMN, there is currently no agreement on surveillance imaging. Most researchers, however, propose annual CT imaging for at least five years. Surveillance may be terminated after 5 years-10 years if the tumor or mucin has not moved beyond the appendix and there is no sign of recurrence. In completely resected LAMN, specific follow-up or surveillance is unnecessary due to the absence of recurrence risk. For T4a LAMN patients, recommendations vary from 6 months to two years of regularly scheduled imaging/tumor marker follow-up, which permits earlier detection of recurrent disease. However, in metastatic (M1a/b) LAMN, baseline imaging was suggested at two months after cytoreductive surgery, then annually for ≥5 years for low-grade peritoneal disease, or every six months for ≥6 years (including chest imaging) for high-grade peritoneal disease. After appendicectomy, the application of colonoscopy is indicated to evaluate for other colonic lesions and to determine if the involvement of the cecum has occurred, which would be consistent with local invasion from an adenocarcinoma.
In conclusion, despite the rare incidence of LAMN, early diagnosis and proper management can increase the survival rate and reduce the risk of PMP. The U/S, CT, MRI, and colonoscopy are valuable in detecting LAMN and its consequences. The gold standard in treating LAMN cases is surgical resection. Evidence supporting right hemicolectomy is very limited and against global recommendations. Regular surveillance is only required in metastatic cases and for a specific period. Post-operative colonoscopy is valuable in detecting other lesions or metastasis.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hassan S, Singh P (2023) The Extent of Resection and Survellience in Appendiceal Mucinous Neoplasm: A Case Report and Review of the Literature. J Clin Trials. 13:527.
Received: 25-Nov-2022, Manuscript No. JCTR-22-20439; Editor assigned: 28-Nov-2022, Pre QC No. JCTR-22-20439 (PQ); Reviewed: 12-Dec-2022, QC No. JCTR-22-20439; Revised: 10-Feb-2023, Manuscript No. JCTR-22-20439 (R); Published: 17-Feb-2023 , DOI: 10.35248/2167-0870.23.13.527
Copyright: © 2023 Hassan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.