
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Mini Review - (2021)Volume 12, Issue 2
Two actin isoforms build the actin cytoskeleton in non-muscle cells: β-and γ-cytoplasmic actins (β- and γ-actins), encoded by ACTB and ACTG1 genes respectively. They are ubiquitously expressed in the different cells in vivo and in vitro, and the b/γ-actin ratio depends on the cell type. Cytoplasmic actins are both essential for the cell survival, but they perform various functions in the interphase and during cell division, as well as they play different roles in neoplastic transformation. In this review, we briefly summarize the research results of recent years and describe the features of the cytoplasmic actins: spatial organization in close connection with their functional activity during the cell cycle.
non-muscle actin isoforms; β-actin; γ-actin
Actin is one of the most abundant proteins in eukaryotic cells. The ability of actin to polymerize and interact with enormous number of other proteins allows it to perform many different functions. All the cytoskeletal proteins are capable of polymerization, however the exchange between monomeric and polymerized actin in the cytoplasm occurs rapidly, the exchange time at the edge of the cell is several seconds. The high dynamics of actin network provides the ability to play multiple roles in the cell mobility, maintaining cell shape, signal transduction, cell adhesion, transcription and muscle contraction, and the formation of a contractile ring during cytokinesis [1,2].
There are six actin isoforms in the cells of vertebrates-four predominantly tissue-specific muscle isoforms (skeletal, cardiac, and smooth muscles), and two non-muscle isoforms (named cytoplasmic β- and γ-actins) that are essential for almost all the cells [3,4]. The ratio and subcellular distribution of actin isoforms are variable and depend on the cell type [5-7].
Despite the long research history, it is still not clear why are two such similar non-muscle actin isoforms coexist in the cell. The following questions remain open: (1) whether non-muscle actins have distinct functions in the cytoplasm of the same cell and at the different stages of the cell cycle; (2) is the localization of non-muscle actins essential for the normal architecture of the cytoskeleton and organization of the intracellular space; (3) what is the contribution of non-muscle actins to the progression of the pathological processes, including the cell transformation?
The family of actin proteins is highly conserved. The greatest difference is observed in the amino acid sequence of muscle and non-muscle isoforms [4]. Two cytoplasmic actins and many actin-binding proteins, which provide the organization of various structures, form the microfilament cytoskeleton of non-muscle cells. The amino acid sequences of cytoplasmic β- and γ-actin (β- and γ-actin hereafter) differ only in four residues located at the N-terminus of the polypeptide chain [3].
β-Actin gene is essential for the survival during embryonic development of mammals. Embryos of mice lacking β-actin are much smaller in size and die at the early stages of development [8]. Embryos without γ-actin pass the prenatal period of development with some delay, and after the birth they experience an increased mortality rate [9]. Mouse embryonic fibroblasts with β-actin knockout show reduced motility compared to normal cells [8]. There is a pronounced compensatory expression of α-smooth muscle actin and activation of the Rho signaling pathway in these fibroblasts. ROCK inhibitors restore motility of cells without β-actin. γ-Actin is an important structural element and positive regulator of cell migration. Knockdown of γ-actin leads to excessive phosphorylation of cofilin and myosin light chain, which indicates ROCK activation, increased contractility, and inhibition of the cell motility [10-13]. Morphological studies of the structures that are formed by non-muscle actins became possible due to highly specific monoclonal antibodies against β- and γ-actin and the method of confocal microscopy [10]. In the non-muscle cells of different origin (epithelial, endothelial, fibroblasts) β- and γ-actin organize different cytoskeletal structures, diversely located within the cell, and can perform distinct functions [10,11,14]. In fibroblasts, β-actin is predominantly located in the stress fibers and in the focal contacts area; cortical and lamellar branched actin network consists of γ-actin (Figures 1A and B). In the lamellipodia, the colocalization of β- and γ-actin is observed (Figure 1B). β-Actin filaments are involved in the processes of cell contraction. In the epithelial cells, β-actin forms basal microfilament bundles and participates in the adhesion junctions; γ -actin organizes the cortical (dorsal) network of actin filaments and some stress fibers (Figure 1C) [10]. Distribution of β- and γ-actin in the endothelial cells is similar to epithelial cells [15].
β-Actin is important for the structure and functional regulation of the adhesion junctions in epithelial cells and determines an apical-basal cell polarity. γ-Actin is associated with tight junctions in the epithelial cells. Suppression of β-actin causes the loss of intercellular contacts in the epithelial cells, while a downregulation of γ-actin induces an epithelial-myofibroblast transition (EMyT), accompanied by an increase of stress fibrils and enhanced expression of α-smooth muscle actin [11,14].
Cytoplasmic β- and γ-actins are also involved in the endothelial barrier function [16]. β-Actin is found mainly in the stress fibers, while γ -actin forms a branched cortical network in the interphase endothelial cells of large vessels [17]. Coordinated rearrangements of β- and γ-actin filaments contribute significantly to the development of endothelial microparticles [15]. Endothelial microparticles are membrane vesicular structures released upon endothelial cell activation or induction of apoptosis [18,19]. Both actin isoforms are responsible for the endothelial barrier function and the dynamics of cell contacts. However, β-actin is vital for endothelial cells: β-actin knockout leads to the death of almost all endothelial cells [20]. The connection of the actin structures with microtubules (MT) is important for the functional activity of endothelial cells. Microfilament-MT interaction provides compression and relaxation of the cell during the endothelial barrier function. Thrombin or nocodazole treatment impairs the barrier function, induces MT disassembly, and leads to the formation of large stress fibers [21]. MT affect the actin filament organization via local changes of actomyosin contractility at the end of stress fibers [22]. Actin filaments interact with the dynamic MT [21,23] which are the majority in endothelial cells [21]. The dynamics of MT is γ-actin-depended, which suggests the presence of a mechanical joint between γ-actin and MT [24]. This joint is possibly established through the intermediates, which may be isoform-specific. For example, MT plus-end binding protein EB1 is known to interact mainly with γ-, but not β-actin [25].
Cytoplasmic actins are segregated in anaphase-telophase of normal mitotic epithelial cells [10,26]. The organization and functions of β- and γ-actins at different phases of mitosis of non-tumor epithelial cells were studied using laser scanning microscopy (LSM) [26]. It was shown that β- and γ-actins are spatially separated in early prophase, anaphase, telophase and during cytokinesis (Figures 1D and 1E). A decrease in β- or γ-actin expression via small interference RNAs (miRNAs) leads to a significant reduction of cell population. A decrease of β-actin causes the generation of multinucleated cells, which indicates a possible cytokinesis failure in these cells. Suppression of γ-actin expression diminishes the number of mitoses. The interdependence between actin isoforms and the MT system during mitosis is observed: Reduction of γ-actin induces the disorganization of the mitotic spindle, and suppression of tubulin polymerization influences β-actin arrangement. Thus, both actin isoforms are required for normal cell division, but each isoform has its specific contribution to this process.
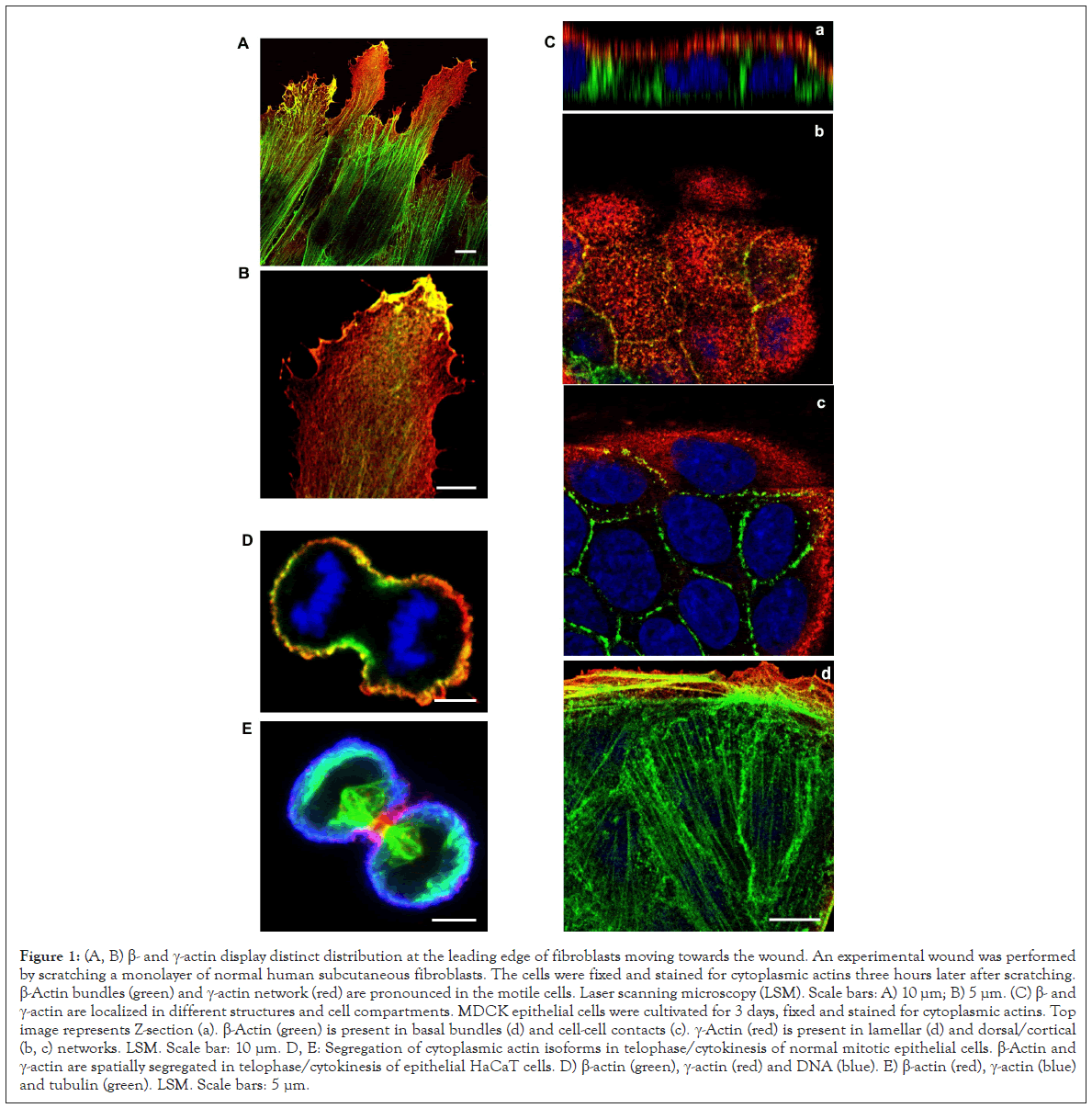
Figure 1: (A, B) β- and γ-actin display distinct distribution at the leading edge of fibroblasts moving towards the wound. An experimental wound was performed by scratching a monolayer of normal human subcutaneous fibroblasts. The cells were fixed and stained for cytoplasmic actins three hours later after scratching. β-Actin bundles (green) and γ-actin network (red) are pronounced in the motile cells. Laser scanning microscopy (LSM). Scale bars: A) 10 μm; B) 5 μm. (C) β- and γ-actin are localized in different structures and cell compartments. MDCK epithelial cells were cultivated for 3 days, fixed and stained for cytoplasmic actins. Top image represents Z-section (a). β-Actin (green) is present in basal bundles (d) and cell-cell contacts (c). γ-Actin (red) is present in lamellar (d) and dorsal/cortical (b, c) networks. LSM. Scale bar: 10 μm. D, E: Segregation of cytoplasmic actin isoforms in telophase/cytokinesis of normal mitotic epithelial cells. β-Actin and γ-actin are spatially segregated in telophase/cytokinesis of epithelial HaCaT cells. D) β-actin (green), γ-actin (red) and DNA (blue). E) β-actin (red), γ-actin (blue) and tubulin (green). LSM. Scale bars: 5 μm.
Actin cytoskeleton is reorganized during tumor transformation. This reorganization provides motility, invasion, and metastasis of tumor cells. Cytoplasmic actins play different roles in neoplastic transformation. The predominance of β-actin induces epithelial differentiation, suppresses cell growth, invasion and tumor growth of colon and lung carcinoma cells. Thereby β-actin acts as a tumor suppressor in the epithelial tumor cells. On the contrary, the dominance of γ-actin enhances malignant features of epithelial tumor cells [27]. The depletion of each cytoplasmic actin leads to impaired proliferation/cell cycle in carcinoma cells [27,28].
The cytoplasmic actins play distinct roles in cell cycle regulation of breast cancer cells. Down-regulation of each cytoplasmic actin isoform inhibits the proliferation of breast cancer cells, but only suppression of β-actin stimulates expression of cyclins A2, B1 and D3, whereas suppression of γ-actin reduces expression of these cyclins. γ-Actin is co-localized with extracellular signal-regulated kinases 1/2 (ERK1/2) in breast cancer MCF7 cells. Reduction of β-actin induces ERK1/2 activation, while γ-actin down-regulation inhibits phosphorylation of ERK1/2. ERK1/2, γ-actin and cyclin A2 directly interact in the same protein complex. Reduction of γ-actin leads to a decrease of cyclin A2, inhibits ERK1/2 signaling and cell proliferation [27-37].
The functions of non-muscle β- and γ-actins have been investigated for a long time, using the molecular and cell biology methods, in particular silencing or overexpression of genes encoding these isoforms. The data have been frequently contradictory, which could be associated with technical difficulties of experiments, including a different level of selective suppression or overexpression of each actin isoform.
Today we consider that β- and γ-actins form distinct structures in the cytoplasm of non-muscle cells of various origins. The distribution within the cell and functions of isoform-specific actin structures are distinguishable at the different stages of the cell cycle-in interphase, and during mitosis. The differences in the polymerization of nonmuscle actins, isoform-specific interaction with proteins that regulate polymerization, allow to suppose that cytoplasmic actins perform distinct functions in the cell. The contribution of actin isoforms to the development of pathological processes, including tumor transformation, is different.
Both cytoplasmic actin isoforms are fundamentally important for the vital activity of the cells, and each isoform has the specific functions, despite the minimal structural differences. The ratio and interaction of actin isoforms are necessary for the normal functioning of interphase and mitotic cells, and imbalance leads to the development of pathologies, including tumor transformation.
This work was supported by the Russian Foundation for Basic Research (grant # 18-29-09082) and was partially supported by Lomonosov Moscow State University Development program (PNR5.13). The authors acknowledge the support by Nikon Center of Excellence at Belozersky Institute of Physico-Chemical Biology."
Citation: Dugina VB, Shagieva GS, Shakhov AS, Alieva IB (2021) The Features of the Cytoplasmic Actins: Cytoskeletal Structures and Functional Activities. J Clin Cell Immunol. 12:614.
Received: 15-Mar-2021 Accepted: 29-Mar-2021 Published: 06-Apr-2021 , DOI: 10.35248/2155-9899.21.12.614
Copyright: © 2021 Dugina VB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This work was supported by the Russian Foundation for Basic Research (grant # 18-29-09082) and was partially supported by Lomonosov Moscow State University Development program (PNR5.13). The authors acknowledge the support by Nikon Center of Excellence at Belozersky Institute of Physico-Chemical Biology."