Research Article - (2016) Volume 2, Issue 4
The Hypoglycaemic Effect of the Components from Pleurotus eryngii was evaluated on model In vitro and In vivo
*Corresponding Author: Yutian Pan, Engineering Technological Center of Mushroom Industry, Minnan Normal University, Zhangzhou, 363000, China, Tel: +86-0596-2523230, Fax: +86-0596-2523230 Email:
Abstract
Four structural substances were extracted from P. eryngii, including PEE-1, PEE-2, PEE-3 and PEE-4. The chemical analysis was conducted to measure the contents of polysaccharide and protein in PEE-1, results found that they were 10.78% and 26.66% respectively. PEE-2, PEE-3 and PEE-4 were respectively identified as β-glucan, chitosan and D-glucosamine hydrochloride by FT-IR, GC, HPLC and HPLC-MS. The results showed that PEE-1, PEE-2 and PEE-3 could inhibit the activity of α-glycosidase from the rabbit isolated small intestine, while the PEE-4 had no inhibitory effect. At the same concentration, the PEE-1 showed more significantly inhibitory effects on α- glycosidase activity than Glucobay (P<0.05). The hypoglycemic effect of PEE-1 had significant differences in the blood glucose between each group and model control group (P<0.05). In glucose tolerance test, the other groups had significant differences compared with the model control group (P<0.05). In addition, high dose of PEE-1 normal group had no significant effect on the fasting blood glucose level with normal control group (P>0.05). Results showed that PEE-1 had the hypoglycaemic function in hyperglycaemia animals, and it could be applied in the research and production of hypoglycaemic health food.
Keywords: Pleurotus eryngii; Polysaccharide; α-glucosidase; Hypoglycaemic
Introduction
Diabetes, cardiovascular disease (CVD) and cancer are the world's three major diseases, and the main features of diabetes are hyperglycaemia and glycosuria. At present, there are more than 120 million patients with diabetes in the world, and most of them are non- Independent diabetes mellitus (NIDDM), i.e. type II diabetes. CVD is the most common and fatal complication in patients with type II diabetes [1]. As a major metabolic abnormalities of diabetes, hyperglycaemia can cause surrounding tissues and organ damage extensively, and participate in the formation of chronic complications of diabetes, cause CVD, hypertension, lower limb necrosis, renal failure, even death and disability. Treatment of diabetes is closely related to hypoglycaemic, and the risk of CVD is significantly reduced by tight control of hyperglycaemia and dyslipidaemia [2]. At present, the chemical drugs were the main treatment of diabetes, while longterm use of chemical drugs would cause a variety of adverse effects. New therapeutic drugs and methods for diabetes will be urgently needed, in which the development of hypoglycaemic drugs with good efficacy is currently becoming a research focus.
Recently, edible fungus have been used for preventing metabolic syndrome as they are economical and largely free from adverse side effects, especially Pleurotus eryngii, and it has been reported to have a good hypoglycaemic effect [3-5]. P. eryngii is the largest species in the oyster mushroom genus. Studies reported that the fruiting body of P. eryngii contained polysaccharides, lipids, peptides, sterols, and dietary fibre, and can affect lipid metabolism, having therapeutic effects on fatty liver in rats [6].
The PEP, a heteropolysaccharide from the fruiting body of P. eryngii , was capable to attenuate the development of insulin resistance and oxidative stress in HF-fed mice, this finding opened the possibility of exploiting functional polysaccharide PEP as the novel preventive and therapeutic ingredients for the mitigation of oxidative stress-induced liver injury [3].
Even though the P. eryngii contains a variety of natural active ingredients with nutritional and healthy functions, the hypoglycaemic components of P. eryngii is lack of systematic screening at present. In this study, four structural materials were gradually separated from the fruiting body of P. eryngii , and they were analyzed and identified by a series of chemical analysis methods. Detected their inhibitory activity on α-glucosidase and found the best one in vitro , and then its hypoglycaemic effect was further studied in vivo . The aim of this study was to provide the reference for the development of hypoglycaemic products and further research on P. eryngii.
Materials and methods
Chemicals
P. eryngii was provided by Fujian Keren Biological Technology Co. Ltd, China, and identified according to the standard of Pharmacopeia of the People's Republic of China. Sample was deposited at the Engineering Technological Center of Mushroom Industry, Minnan Normal University, China.
4-Nitrophenyl-α-D-glucopyranoside was purchased from Merck, Germany; Glutathione, Alloxan monohydrate was purchased from Sigma, USA; D-Glucosamine Hydrochloride, Chitosan, Rhamnose, Arabinose, Xylose, Glucose and Mannose standards were purchased from Sigma; Glucobay (Acarbose Tablets) was purchased from Bayer, Germany; FreeStyle Lite Blood Glucose Test Strips was purchased from Abbott Diabetes Care Inc, USA; Dextran T-40 standard was purchased from Pharmacia, USA.
Preparation of P. eryngii structural materials
P. eryngii extract 1 (PEE-1): The dry powders of P. eryngii fruit body were extracted in hot water (w:w 1:40) at 100°C for 2 hour. After cooling and centrifugating, the supernatant fraction was concentrated to 5°C at least. At last, the concentrate was lyophilized obtaining the P. eryngii extract 1 (PEE-1).
P. eryngii extract 2 (PEE-2): The residue after extracting PEE-1 was extracted continually in 2% NaOH solution (w: w 1:3) at 100for 2 hour. After continuous stirring then the extracted solution was filtered. The new residue was washed to neutral with RO water and then lyophilized was crushed into powders. And the powders was extracted in hot water (w: w 1:40) at 100 ˚ for 2 hour. After cooling and centrifugating, the supernatant was filtered with the ultrafiltration membrane of molecular weight 100 Kda (Vivaflow 200, Sartorius, Germany) to obtain interception liquid that was concentrated and ≥ 5°C. At last, the concentrate was lyophilized obtaining the P. eryngii 2(PEE-2).
P. eryngii extract 3 (PEE-3): The dry powders of the residue after extracting PEE-2 were extracted in 45% NaOH solution (w:w 1:10) at 100°C for 6 hour. After cooling, the extracted solution was filtered. And the filter residue washed to neutral with RO water, and extracted in 1% CH3COOH solution (w:w 1:10) at 80°C for 2 hour after continuous stirring. After cooling and centrifugation, the supernatant fraction was adjusted to pH ≥ 10 with 1 mol/L NaOH solution and stayed for 4 hour. After filtering, the precipitation washed with RO water for 3-5 times was lyophilized obtaining the P. eryngii extract 3 (PEE-3).
P. eryngii extract 4 (PEE-4): PEE-4 was prepared from dry powders of the residue after extracting PEE-3. It was treated with 36% HCl at the ratio of the material and solution 1:4, hydrolyzed under 90°C for 4 hour. And then it was decolorized using 2% active carbon at 80°C for 120 min. After decolorization, the hydrolysate was concentrated, crystallized, recrystallized, washed and dried to obtain PEE-4.
Chemical analysis
Chemical composition analysis of PEE-1: The contents of protein, total sugar, polysaccharide, reducing sugar, amino nitrogen, ash, Pb and as in PEE-1 were detected with the extract of A. bisporus as a comparison.
FT-IR analysis: From 500 cm-1 to 4000 cm-1 was used for the determination of the functional groups present in PEE-2, PEE-3 and PEE-4 on a Nicolet Fourier transform infrared spectrometer (NICOLET iS10, Thermo, USA). 20 mg sample were mixed with KBr (w: w 1:50-100) respectively, and then tableted for infrared spectrum detection.
HPLC chromatographic analysis of PEE-2: HPLC was used to analyze PEEE-2 by an instrument (1200, Agilent, USA) equipped with waters Ultrahydrogel TM 500 column (7.8 mm × 300 mm) and a differential index detector (RID). The column temperature was maintained at 80°C; 20 μl of 1 mg/ml PEE-2 dissolved in ultrapure water was injected into the column, eluted with ultrapure water at a flow rate of 0.6 ml/min.
GC chromatographic analysis of PEE-2: The 20 mg PEE-2 was hydrolyzed to release constituent monosaccharides with 2 mL of 2 M TFA at 120°C for 3 h in a 5 mL sealed ampoule. The hydrolysate was washed with a little methanol for 3-5 times and then dried every time to remove the TFA completely. The hydrolysate was treated with 1 ml pyridine and 10 mg hydroxylamine hydrochloride. The solution reacted in a shaker at 300 rpm at 90°C for 30 min. After cooling to 25°C, 1 mL acetic anhydride was added for acetylation reaction at 90°C for 30 min. The solution was centrifuged at 12000 rpm for 5 min to obtain supernatants. It was diluted with pyridine (v:v 1:10) and subjected for GC analysis. With rhamnose, arabinose, xylose, glucose and mannose as the monosaccharide control standards, these standards were treated as the same as the above methods. GC using an instrument (GC3800, Varian, USA) equipped with an WCOT fused silica capillary column (30 m × 0.25 mm) and a flame-ionization detector (FID), the oven temperature was maintained at 270°C column temperature was maintained at 220°C, detector temperature was maintained at 300°C ; gas flow rate: N2 was 20 ml/min, H2 was 30 ml/ min, Air was 200 ml/min; 0.4 μl test sample was injected into the column one time [7].
HPLC-MS chromatographic analysis of PEE-4: The purity and molecular weight of PEE-4 were determined by a HPLC-MS system (HPLC: Ultimate 3000, Thermo, USA; Mass spectrometer: 6300, Agilent, USA). The HPLC system was fitted with StableBond C18 column (5 μm, 250 mm × 4.6 mm) and a RI detector, detection wavelength 195 nm. Column and detector temperatures were maintained at 30°C. 20 μl of 1.0 mgml PEE-4 or GAH standard dissolved in ultrapure water were injected into the column, eluted with PBS mixed acetonitrile (v: v 60:40) at a flow rate of 1.0 ml/min. Ion source: electrospray ionization, negative ion mode (ESI-). Capillary voltage: 3.0 kV. Ion source temperature: 120°C. Desolvation gas: N2. Desolvation temperature: 350°C. Desolvation gas flow rate: 750 L/hour. Collision gas: high purity Ar. Scanning mode: multiple reaction monitoring (MRM) [8].
Effects of P. eryngii extracts on α-glucosidase
Preparation of α-glucosidase: After 24 hour of fasting, the male adult rabbit were killed with air embolism method. The 10 cm length samples at the upper end of the small intestine were immediately taken and washed several times with cold saline, shredded and added into 4 0.1 mol/L pH 6.8 PBS (w: w 1:5), then homogenated at 10000 rpm for 1-2 min for several times. The homogenate was centrifuged at 4000 rpm in 4°C for 20 min to obtain the supernatant containing α- glucosidase. The supernatant was placed in -20°C for testing later.
The α-glucosidase activities after treatment of P. eryngii extracts: PEE-1, PEE-2, PEE-3, PEE-4 and Glucobay were prepared into 0 mg/mL, 2.5 mg/ml, 5 mg/ml, 10 mg/mL testing solution using 0.1 mol/L pH 6.8 PBS. In 96-wellplate, each well was mixed with 100 μl testing solution, 10 μl supernatant from the rabbit isolated small intestine and 50 μl 1 mg/ml glutathione, and each testing solution repeated 8 wells. The reaction system was heated at 37°C for 10 min, and then 50 μl 29 mmol/L PNPG was added into each hole and continued to heat at 37°C for 10 min. At last, 100 μl 0.2 mol/l Na2CO3 was added into each hole. The absorbance was determined at wavelength under 405 nm.
Inhibition rate of α-glucosidase=[(A0-A0')-(A-A')]/(A0-A0') × 100%. Where: A0— The reaction system was not added into testing solution, α-glucosidase was not inactivated by heating; A0'— The reaction system was not added into testing solution, α-glucosidase was inactivated by heating at 95°C for 10 min; A— The reaction system was added into testing solution, α-glucosidase was not inactivated by heating; A'— The reaction system was added into testing solution, α- glucosidase was inactivated by heating 95˚C for 10 min.
Effects of PEE-1 in mice
Animals: SPF male Kunming mice weighing 26 ± 2 g were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (China quality certification number: SCXK (Hu) 2012-0002). Feed was obtained from the same company. Water was RO water prepared by our laboratory.
Animal was brought at a level 100000 animal rooms for cleanness which was in a controlled environment (22 ± 2°C, 60 ± 5% relative humidity). All animal protocols were approved by our animal care and committee.
Preparation of hyperglycaemia animal model and experimental design: 20 mice were fasted for 5 h randomly and their fasting blood glucose was determined as the basic blood glucose level. Then mice were fasted for 24 h and hyperglycaemia animal model was made by alloxan injected from tail vein (45 mg/kg.bw). After 5 days, fasting blood glucose was determined. 40 successful model animals which had 10-25 mmol/L of blood glucose were chosen and randomly divided into one model control and three dose groups. Each group had 10 mice and the difference of blood glucose among groups was below 1.1 mmo1/L. another 10 normal mice were as normal control group. Three doses of PEE-1 groups were 0.25 g/kg.bw, 0.50 g/kg.bw and 1.50 g/ kg.bw, respectively.
Hypoglycaemic test: As the standard of 20 ml/kg.bw of gavage volume in mice, the concentrations of PEE-1 in three dose groups were prepared in 12.50 mg/ml, 25.00 mg/ml and 50.00 mg/ml, respectively. Mouse was given by gavage once a day for 30 days, with equal volume distilled water for control group.
Mice were fasted for 5 hour and body weights were weighed and recorded every day. After 30 days, fasting blood glucose was determined. The differences among all groups and the decline at the beginning and end of the experiment were compared. The decline percentage of blood glucose=(blood glucose at the beginning of the experiment–blood glucose at the end of the experiment)/blood glucose at the beginning of the experiment × 100%.
Glucose tolerance test: fasting blood glucose determined at the end of the above experiment was as the initial value (0 hour). Different concentrations of PEE-1 were given to the dose groups and the same volume water was given to the control group. After 15 min, 2.0 g/kg.bw of glucose was given orally. Blood glucose was determined after 0.5 and 2 hour. The change of blood glucose at the 0, 0.5th and 2nd hour in all groups was observed.
Glucose tolerance (the area under the concentration-by-time curve)=0.25 × (blood glucose at 0 hour+4 × blood glucose at 0.5th h+3 × blood glucose at 2nd hour)
Statistical analysis
All data were analyzed by SPSS 19.0. The possible association among groups was compared using a χ2 test or Fisher's exact test. Differences with a p-value less than 0.05 were considered to be statistically significant.
Results
Chemical analysis results
Chemical analysis results of PEE-1: 270 g dry powders of PEE-1 were extracted from 1 kg dry fruiting bodies of P. eryngii with hot water, and the chemical components of PEE-1 were listed in Table 1. Without any separation and purification, the natural polysaccharide content in PEE-1 reached 10.78%. Comparison of the test results between P. eryngii and A. bisporus , the content of polysaccharide in PEE-1 was 107.31% higher than the extract of A. bisporus, and the content of total sugar in PEE-1 was more than 3 times as much as the extract of A. bisporus . However, both of the reducing sugars were not detected.
| Test item | Protein(%) | total sugar(%) | polysaccharide(%) | Reducing sugar (%) | Amino nitrogen (g/100 g) | Ash(%) | Pb (ppm) | As (ppm) | |
|---|---|---|---|---|---|---|---|---|---|
| Variety | P. eryngii | 26.66 | 49.45 | 10.78 | not detected | 0.96 | 11.8 | 0.14 | 0.28 |
| A. bisporus | 29.85 | 14.67 | 5.2 | not detected | 2.21 | 15.86 | 0.64 | 0.79 |
Table 1: Chemical components of PEE-1 in two varieties.
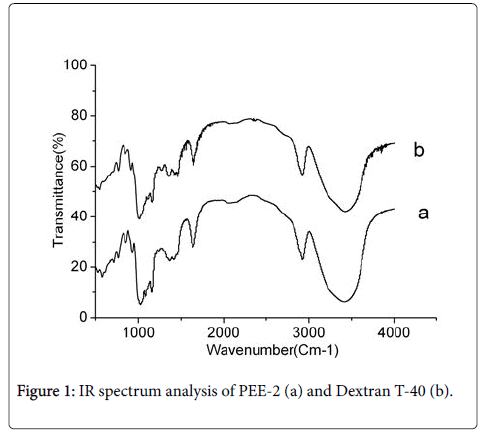
Chemical analysis of PEE-2: In the experiment, 3.5 g dry powders of PEE-2 were extracted from 1 kg dry fruiting bodies of P. eryngii . The FT-IR analysis ranging from 4000 cm-1 to 500 cm-1 showed that PEE-2 had the characteristic absorption bands of polysaccharide (Figure 1a) at 1000 ~ 1100 cm-1, 1400 ~ 1530 cm-1, 2800 ~ 2900 cm-1, 3100 ~ 3500 cm-1.
The absorption band at 3447 cm-1 was O-H stretching; the absorption band at 2927 cm-1 was C-H stretching; the absorption band at 1078 cm-1 was produced by pyran ring lactone and hydroxy, it was the typical infrared spectrum signal of glucan. The absorption band at 854 cm-1 was variable angle vibration absorption peak of the differential phase C-H of beta end of glucose, it indicated the presence of beta glycosidic bonds in PEE-2. Moreover, the PEE-2 had a highly similar infrared spectrum absorption bands (Figure 1a) with the standard β-dextran (Figure 1b), this result showed that the PEE-2 was β-polysaccharide.
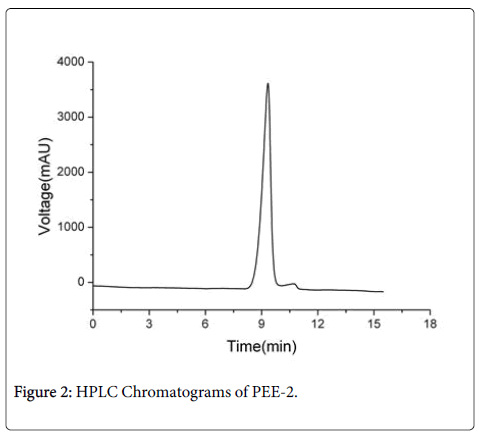
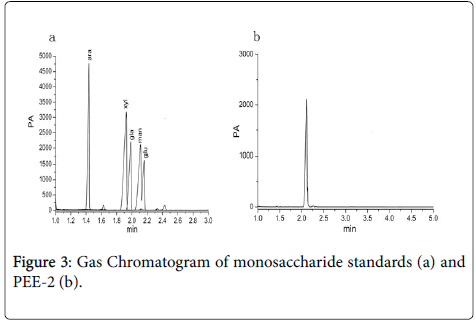
The purity of the PEE-2 was analyzed using HPLC, only one chromatographic peak was appeared in HPLC map (Figure 2), the peak shape was symmetrical and was characterized as Gauss. The result showed that the average relative molecular weight of the PEE-2 was relatively uniform. Monosaccharide composition analysis of PEE-2 by GC, there was only one chromatographic peak at 2.11 min (Figure 3a), the retention time of the peak was consistent with the glucose standard (Figure 3b). These results showed that the PEE-2 was composed of only glucose through beta glycosidic bond, it would be judged that the PEE-2 was β-glucan.
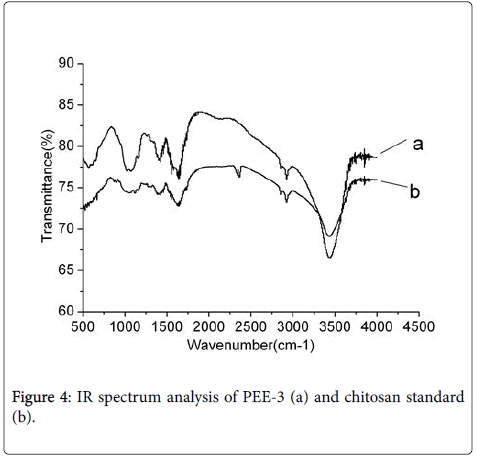
Chemical analysis of PEE-3: The FT-IR analysis ranging from 4000 cm-1 to 500 cm-1 showed that PEE-3 had a highly similar carbohydrate pattern with the chitosan standard (Figure 4). The strong absorption bands near 3442 cm-1 were for intermolecular O-H and CH2OH hydrogen bonds or O-H stretching. The absorption bands near 2926 cmcm-1 were C-H stretching, The absorption bands near 2885 cm-1 were C-H stretching, The absorption band near 1650 cm-1 was C=O-NHCH3 stretching and it was the characteristic bands of amides bands. The absorption bands near 1384 cm-1 were C-H bending and C-CH3 deformation mode. The absorption bands near 1084 cm-1 were C-O-C stretching. The result showed that the structure of the PEE-3 was consistent with the chitosan standard.
In addition, according to the literature to test sample [9], the degree of deacetylation (DD) of the PEE-3 was 93.5%, it belonged to the high degree of deacetylation chitosan product.
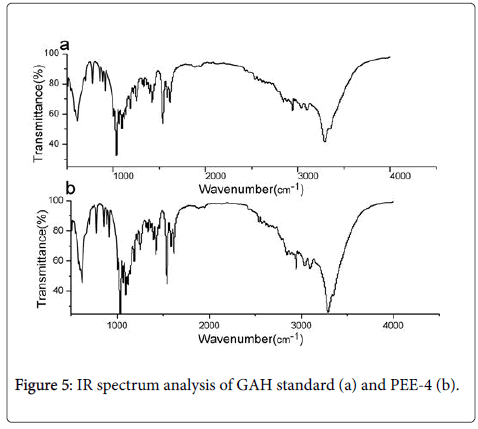
Chemical analysis of PEE-4: In the experiment, the extraction rate of PEE-4 from the dry fruiting bodies of P. eryngii was 2.46%. The FTIR analysis ranging from 4000 cm-1 to 500 cm-1 showed that PEE-4 had a highly similar carbohydrate pattern with the D-glucosamine hydrochloride (GAH) standard (Figure 5). The PEE-4 sample had the same characteristic absorption bands at 3307 cm-1 3094 cm-11622 cm-11536cm-11426 cm-11095 cm-1910 cm-1 with GAH standard.
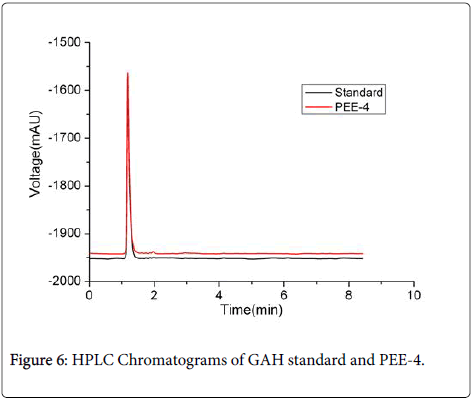
The HPLC analysis (Figure 6) showed that 20 μL 1.0 mgml of PEE-4 and GAH standard had the same symmetrical peaks at 1.19 min with the peak area of 1.92 × 103 mAUs. This result indicated that PEE-4 was a homogenous sample with no other peak. The PEE-4 was further confirmed to be D-glucosamine hydrochloride (GAH) by analyzing its mass spectrum data (PEE-4: m/z 215.1 [M-H+]; standard: m/z 251.1.1 [M-H+]).
The activities of α-glucosidase
The results (Table 2) showed that Glucobay could obviously inhibit α-glucosidase activity, and the inhibition rate increased with the concentration increasing. So we thought that the inhibitory activity model was reliable by the α-glucosidase from the rabbit isolated small intestine. On this basis, we detected the inhibitory effects of 4 different extracts of P. eryngii to α-glucosidase, and the results showed that PEE-1, PEE-2 and PEE-3 could inhibit α-glycosidase activity, and the inhibition rate increased with the concentration increasing. The PEE-1 showed more significant inhibitory effects on α-glycosidase activity than Glucobay at the same concentration (at 2.5 mg/ml and 5 mg/ml: p<0.01; at 10 mg/ml: p<0.05). PEE-2 didn’t constantly inhibit the activity of α-glucosidase, its inhibition rate was only 20.7% of PEE-1 at 10 mg/ml. Precipitation was found in the PEE-3 reaction system, and we considered the reason might be that the reaction system had an alkaline compound Na2CO3 that leaded chitosan (PEE-3) to precipitation, so the test result of PEE-3 was not reliable by this reaction system. PEE-4 did not inhibit the α-glucosidase activity. The results indicated that PEE-1 had an important value for development of hypoglycaemic products.
| Sample | Inhibition rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 2.5 mg/ml | 5 mg/ml | 10 mg/ml | ||||
| △`A0 | △`A2.5 mg/ml | Inhibition rate (%) | △`A5 mg/ml | Inhibition rate (%) | △`A10 mg/ml | Inhibition rate (%) | |
| PEE-1 | 1.92±0.06 | 1.30±0.01 | 32.49±2.23** | 1.18±0.06 | 38.48±4.08** | 1.02±0.06 | 46.84±3.47* |
| PEE-2 | 1.88±0.03 | 1.96±3.54 | 1.88±0.10 | 2.22±5.12 | 1.74±0.01 | 9.70±2.91 | |
| PEE-3 | 1.98±0.04 | -3.14±3.69 | 1.38±0.01 | 28.24±2.45* | 0.61±0.01 | 68.15±1.36*** | |
| PEE-4 | 1.95±0.08 | -1.71±7.06 | 1.98±0.06 | -3.16±6.64 | 1.99±0.07 | -3.70±6.94 | |
| Glucobay | 1.86±0.08 | 3.00±5.99 | 1.72±0.07 | 10.30±6.50 | 1.25±0.04 | 34.84±3.18 | |
Table 2: Inhibitory effects of four extracts of P. eryngii on the α- glucosidase in the rabbit small intestine of 4 extracts (x ± s; n=8 ) ( ***p<0.001, **p<0.01, *p<0.05, all relative to the Glucobay).
Effects of PEE-1 on the body weight in each group
The results (Table 3) showed that there was no significant difference in body weight between HD normal group and normal group (P>0.05) within 30 days. Compared with normal group, the model group and all dose groups showed significantly decreased in the body weight (P<0.001) at 15th day and 30th day, which showed that hyperglycaemia could severely affect the body weight of animals. Compared with model group, the each dose group showed no significant difference in the body weight (P>0.05) within 30 days, which showed that PEE-1 couldn’t effectively prevent the abnormal change in the body weight of hyperglycaemia mice.
| Group | Body weight (g) | ||
|---|---|---|---|
| 1st day | 15th day | 30th day | |
| Normal control | 24.87 ± 0.56 | 32.74 ± 1.06 | 35.83 ± 1.30 |
| Normal+HD | 24.95 ± 0.73 | 32.96 ± 0.98 | 35.52 ± 1.21 |
| Model control | 24.89 ± 0.53 | 25.96 ± 1.15*** | 26.82 ± 1.20*** |
| Model+LD | 25.08 ± 0.55 | 25.87 ± 1.27*** | 27.11 ± 1.10*** |
| Model+MD | 24.91 ± 0.54 | 27.02 ± 1.18*** | 27.18 ± 1.39*** |
| Model+HD | 25.31 ± 0.44 | 27.57 ± 1.50*** | 27.75 ± 1.30*** |
Table 3: Effects of PEE-1 to the body weight in each group (x ± s; n=10 )(***p<0.001, relative to the Normal group, Model: hyperglycemia mice; LD: Low dose, 0.25 g/kg.bw; MD: Medium dose, 0.50 g/kg.bw; HD: High dose, 1.50 g/kg.bw).
Effects of PEE-1 on the fasting blood glucose levels in each group
The results were listed in Table 4. Compared with normal control group, the high dose PEE-1 showed no significant difference in fasting blood glucose levels and the decline percentages of the fasting blood glucose levels after gavage for 30 days (P>0.05). The result showed that the PEE-1 had no effect on the fasting blood glucose levels in normal animals.
| Group | Blood glucose at the beginning of the experiment (mmol/L) |
Blood glucose at the end of the experiment (mmol/L) |
The decline percentage of blood glucose (%) |
|---|---|---|---|
| Normal control | 4.92 ± 0.33 | 5.07 ± 0.29 | -3.41 ± 8.41 |
| Normal+HD | 4.92 ± 0.32 | 5.04 ± 0.34 | -2.91 ± 10.55 |
| Model control | 18.45 ± 0.88 | 16.84 ± 0.85 | 8.73 ± 1.36 |
| Model+LD | 18.56 ± 1.05 | 16.26 ± 1.00 | 12.34 ± 3.95* |
| Model+MD | 18.47 ± 0.76 | 15.93 ± 0.92* | 13.69 ± 4.81* |
| Model+HD | 18.58 ± 0.77 | 15.40 ± 0.98** | 17.12 ± 3.75*** |
Table 4: Effects of PEE-1 on the fasting blood glucose test in each group ( x±s; n=10 )( ***p<0.001, **p<0.01, *p<0.05, all relative to the model control group; Model: hyperglycaemia mice; LD :Low dose, 0.25 g/kg.bw; MD: Medium dose, 0.50 g/kg.bw; HD: High dose, 1.50 g/ kg.bw).
As for model mice, compared with normal group, after gavage using different concentrations of PEE-1 for 30 days, the model group showed significant differences in the fasting blood glucose levels (P<0.01); so did model MD group (P<0.05) and while LD group had no significant difference with normal group (P>0.05). The decline of blood glucose levels in each dose group had a rising trend compared with control and the model HD group showed a very significant difference (P<0.001) and so did model MD and model LD groups (P<0.05).
Effects of PEE-1 on the glucose tolerance in each group
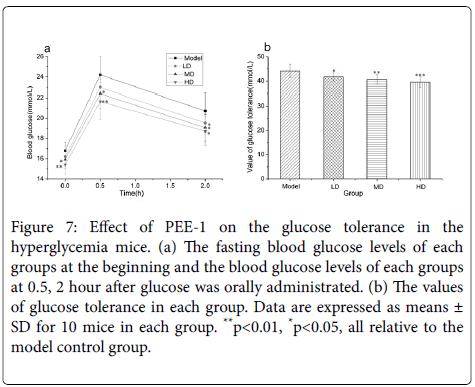
Figure 7 showed the glucose tolerances of the hyperglycaemia model mice. After 2.0 g/kg.bw of glucose was orally administrated, and blood glucose levels in all model dose groups were lower than that in model control group at 0.5, 2 h and decreased with the increase of dose. Model HD and model MD group had significant difference in blood levels at 0.5 and 2 hour (P<0.05). The glucose tolerances of HD and MD group were significantly lower than control group (P<0.01), and so did LD group (P<0.05).
Figure 7: Effect of PEE-1 on the glucose tolerance in the hyperglycemia mice. (a) The fasting blood glucose levels of each groups at the beginning and the blood glucose levels of each groups at 0.5, 2 hour after glucose was orally administrated. (b) The values of glucose tolerance in each group. Data are expressed as means ± SD for 10 mice in each group. **p<0.01, *p<0.05, all relative to the model control group.
Discussion
Mushroom as a kind of higher fungi can be used as food and medicine [10,11]. In recent years, many studies indicated that polysaccharides from mushroom had obvious health care and disease prevention function. The species of polysaccharides of edible fungi are very complex and diverse. Polysaccharides which were distributed in different locations of the cells had different biological functions [12]. In this study, four structural materials were gradually separated from the fruiting body of P. eryngii , including PEE-1, PEE-2, PEE-3 and PEE-4. The chemical analysis was used to measure the contents of polysaccharide and protein in PEE-1, our results found that their contents were 10.78% and 26.66% respectively. Besides, the PEE-2, PEE-3 and PEE-4 were identified as β-glucan, chitosan and Dglucosamine hydrochloride respectively using FT-IR, GC, HPLC, HPLC-MS. The results showed that PEE-1, PEE-2 and PEE-3 could inhibit α-glycosidase activity in vitro, and their inhibition rate increased with the concentration increasing, while PEE-4 had no inhibitory effect. These results proved that polysaccharides distributed in different locations of the cells showed different biological functions, and it provided a valuable reference for screening the effective hypoglycemic components of P. eryngii .
The α-glucosidase from the rabbit isolated small intestine was as the model in vitro , which was existed in animal intestine villi mucosa brush border. It could hydrolyzed the starch and other related polysaccharides into glucose from their non-reducing ends at the α-1-4 glycosides bond. The activity of the α-glucosidase in animal small intestine influenced the absorption and utilization of carbohydrates, for example, sucrose, starch and so on. The characteristics of α- glucosidase of the mammalian are more in line with the human [13]. The Glucobay was as a positive control medicine, because of its unique hypoglycemic mechanism. It was the first inhibitor of the α- glucosidase to be approved for clinical application by FDA, due to its good safety and no toxic effect on the body, Glucobay has always been the first choice of drugs for diabetic [14]. Interestingly, under the same concentration, the PEE-1 showed stronger inhibitory effects on α- glycosidase activity than Glucobay, which suggested that PEE-1 might have much value for further study treating diabetes.
The results in vivo suggested that, each group showed significantly increased in the fasting blood glucose levels compared with the model control group (P<0.05), especially HD group (P<0.001). The glucose tolerance test results showed that HD, MD and LD has dramatically differences compared with the model control group (P<0.01, P<0.01, P<0.05). Compared with normal control group, HD of PEE-1 normal group had no significant effect on the fasting blood glucose levels (P>0.05). According to reported laws and regulations [15], one positive is enough between the fasting blood glucose levels and the glucose tolerance in hyperglycemic mice, and the test sample had no effect on the fasting blood glucose levels in normal animals, it would be able to determined that the test sample had auxiliary effects in reducing blood glucose function of animal experiment. The results showed that two indicators were both positive with the PEE-1, and the PEE-1 had no effect on the fasting blood glucose levels in normal animals. So PEE-1 had the hypoglycaemic function in hyperglycaemia animals, it would be able to applied in the research and production of hypoglycaemic health food.
Conclusions
This study found that PEE-1 showed an inhibiting effect to the hypoglycaemia. Further studies will be carried out, such as hypoglycaemic effects of PEE-2, PEE-3 and PEE-4 in hyperglycaemia animals, and separation accurate and effective hypoglycaemic components in PEE-1.
Acknowledgments
This work received financial supports from Major Projects of Science & Technology, Fujian, P. R. China (2013Y0081) and (2015J05071); National Science & Technology Pillar Program during the Twelfth Five-year Plan Period, P. R. China (2012BAD36B05).
Conflict of Interests
The authors declare that they have no any conflict of interests.
References
- Schreij G, Janknegt R (2007) InforMatrix nucleoside/nucleotide reverse transcriptase inhibitor 'backbones'. Expert Opinion on Pharmacotherapy 8: 37-47.
- Mbikay M (2012) Therapeutic Potential of Moringaoleifera Leaves in Chronic Hyperglycemia and Dyslipidemia: A Review. Front Pharmacol 3: 24.
- Ren D, Zhao Y, Nie Y, Lu X, Sun Y, et al. (2014) Chemical composition of Pleurotuseryngii polysaccharides and their inhibitory effects on high-fructose diet-induced insulin resistance and oxidative stress in mice. Food Funct 5: 2609-2620.
- Li Z, Song T, Feng C (2015) Effect of different storage temperature on hypobaric storage quality of Pleurotuseryngii. NongyeGongchengXuebao/transactions of the Chinese Society of Agricultural Engineering 31: 332-338.
- Seto SW, Lam TY, Tam HL, Au AL, Chan SW, et al.(2009) Novel hypoglycemic effects of Ganodermalucidum water-extract in obese/diabetic (+db/+db) mice. Phytomedicine 16: 426-436.
- Huang JF, Zhan T, Yu XL, He QA, Huang WJ, et al. (2016) Therapeutic effect of Pleurotuseryngii cellulose on experimental fatty liver in rats. Genet. Mol. Res 15: 15017805.
- Huang JF, Ou Y, Yew TW, Liu J, Leng B, et al. (2016) Hepatoprotective effects of polysaccharide isolated from Agaricusbisporus industrial wastewater against CCl4-induced hepatic injury in mice. Int J BiolMacromol 82: 676-686.
- Lin JM, Huang JF, Chen JY, Lin ZC, Ou YX, et al.(2016) Low cytotoxic D-mannitol isolated from the industrial wastewater of Agaricusbisporus. Journal of Food and Nutrition Research 4: 610-614.
- China, T.S.P.C.o.P.R. (2010) Pharmacopoeia of the People's Republic of China. Beijing: Chinese Medical Science Press.
- Fujioka K, Greenway F, Sheard J, Ying Y (2006) The effects of grapefruit on weight and insulin resistance: relationship to the metabolic syndrome. Journal of medicinal food 9: 49-54.
- Liu IM, Tzeng TF, Liou SS, Lan TW (2007) Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci 81: 1479-1488.
- Klis F, Ram A, Groot PD (2007) A Molecular and Genomic View of the Fungal Cell Wall. Springer Berlin Heidelberg 8: 97-120.
- Suresh Babu K, Tiwari AK, Srinivas PV, Ali AZ, China Raju B, et al. (2004) Yeast and mammalian alpha-glucosidase inhibitory constituents from Himalayan rhubarb Rheum emodiWall.exMeisson. Bioorg Med ChemLett 14: 3841-3845.
- Elbein AD (1991) Glycosidase inhibitors as antiviral and/or antitumor agents. Seminars in cell biology 2: 309-317.
- LI B (2005) Health product for assisting blood sugar-decreasing function and its preparation method.
Copyright: © 2016 Lin J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.