Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Short Communication - (2023)Volume 11, Issue 3
The Myelofibrosis (MF) Inflammatory milieu contributes to the development of patient specific systemic symptoms, alterations in tissue specific micro-environments and immune dysfunction which sustain the survival advantage of malignant hematopoietic stem/progenitor cells. Interleukin-8 (IL8) is a chemokine that plays a pivotal role in MF disease progression by not only directly affected malignant hematopoiesis but also the tumor microenvironment and immunity. We presented a series of both in-vitro and in-vivo studies which docum1ent the impact of IL8/CXCR1/2 signaling on MF disease progression and identify this pathway as a therapeutic target for MF patients.
Myelofibrosis; Microenvironment; IL8/CXCL8; CXCR1/2; Reparixin; Immunity
Myelofibrosis (MF) originates at the level of Hematopoietic Stem Cell (HSC/HPC) which possess Myeloproliferative Neoplasms (MPN) specific driver mutations that up-regulates the JAK/STAT signaling pathway [1-3]. MF is also associated with upregulation of NF-κB which results in the generation of an inflammatory milieu which contributes to the development of patient systemic symptoms as well as alterations in tissue specific microenvironments which promote MF HSC predominance [4,5].
Cytokines including Interleukin 6 (IL-6), Transforming Growth Factor Beta (TGF-β), Vascular Endothelial Growth Factor (VEGF) Interleukin 8 (IL-8) and Tumor Necrosis Factor Alpha (TNF-α) are present at increased levels in MF patient plasma [6,7]. Of particular interest is the observation that elevated IL-8 plasma levels are significantly associated with an inferior MF leukemia-free survival [8-10].
IL-8 is produced by many cell types, including macrophages, megakaryocytes, epithelial cells, airway smooth muscle cells, endothelial cells and hematopoietic cells and promotes biological functions involving inflammation, Hematopoietic Stem Cell (HSC) proliferation, mobilization, neo-angiogenesis and immune function by binding to the G protein-coupled serpentine receptors CXCR1 and CXCR2 [11-13]. Frequently, tumors coopt the production of IL-8, which favors tumor progression by promoting tumor angiogenesis, cancer stem cell survival and the attraction of immune suppressor myeloid cells to tumor sites [14,15]. Although IL-8 is not present in the mouse genome, functional homologues of these receptors have been identified with high affinity for the murine counterparts of human IL8.
Janus Kinase2 (JAK2) inhibitory therapy which represents the present standard of care for symptomatic MF patients leads to reduced levels of many pro-inflammatory cytokines with the notable exception of IL8. Furthermore, JAK2 inhibitory therapy is incapable of preventing evolution to more overt phases of MF or secondary acute myeloid leukemia [16]. The confluence of these observations led us to examine the role of IL8 in MF disease progression. Single-cell expression profiling of isolated MPN CD34+ cells from patients with varying degrees of fibrosis revealed an increase in expression of chemokines including IL8 that signal through CXCR2 [17]. Integrated ATAC/RNA sequencing revealed enriched inflammatory pathways, including TNFα/NF-κB and Toll-Like Receptor (TLR) signaling in IL8 secretor vs. non-secretor cells. Gene expression and chromatin accessibility studies identified evidence of neutrophil activation, alarmin over-expression, and acute phase inflammatory responses in IL8-secreting MF CD34+ cells. The frequency of IL8-secreting CD34+ cells was correlated to the degree of reticulin fibrosis and leukocytosis [17].
IL8/CXCR1/2 signaling has been shown to affect the behavior of a human MF malignant blood cells [17]. Both the number of MF CD34+ cells expressing CXCR1/2 and the intensity of expression were enhanced as compared to Normal Donors (ND). Exogenous IL8 enhanced proliferation of MF but not ND CD34 + and differentiation to CD33+ monocytes and CD41+ megakaryocytes. CFU-GM colony output was related to the degree of CXCR1/2 surface receptor expression. In addition, IL8 increased the numbers of JAK2V617F+ colony numbers cloned from MF CD34+ cells. Notably, both CXCR1/2 surface expression and IL8 single-cell cytokine enumeration correlated with the JAK2V617F variant allele frequency which is consistent with recent single cell studies suggesting that the magnitude of JAK/STAT signaling corresponds with IL8/CXCR2 output [17].
Using the human MPLW515L MF mouse model, we documented the enrichment in IL8/CXCR2 signaling and assessed the role of IL8/CXCR2 in MF pathogenesis. These studies were possible due to the close homology murine and human CXCR1/2 share which allows murine cells bind hIL8, and activate similar downstream events [17].
The phenotype of the Cxcr2-/- hMPLW515L mice was associated with normalization of white blood cell and platelet counts and reduced BM and spleen fibrosis as compared to WT Cxcr2f/f-expressing hMPLW515L mice. Treatment with reparixin, an oral CXCR1/2 inhibitor, not only decreased the proliferation of malignant MF cells which were enhanced in the presence of IL8, but also blunted the development of the MF phenotype in hMPLW515L mice. Administration of reparixin and ruxolitinib alone or in combination significantly reduced the degree of leukocytosis, BM megakaryocyte numbers, platelet counts and resulted in a significant reduction in reticulin fibrosis both in the BM and spleen. Reparixin therapy also led to a reduction in bone marrow and spleen fibrosis in another MF mouse model, Gata1low mice. More importantly, minimal toxicity was observed with reparixin monotherapy [17]. These studies established that CXCR1/2 might serve as a potential target for the treatment of MF irrespective of the MPN driver mutation status [17].
Recent attempts to harness the immune system to treat MF have met with futility. MF is associated with a T cell exhaustive state due to persistent tumor induced activation by neoantigens and elevated levels of a number of cytokines including IL-6, TGF-β, IL1 beta, TNF and IL8 which are known to act as chemo attractants and activators of immunosuppressive leukocytes [18]. Levels of Vascular Endothelial Cell Growth Factor (VEGF) are also elevated in MF and lead to increased micro vessel density and splenic neo angiogenesis which is also contributes to immune dysfunction. Inhibition of the VEGF/VEGFR pathway has the capability to promote vascular normalization, increase the intra-tumor infiltration of lymphocytes, and decrease the number and function of inhibitory immune cell types, IL8 upregulates VEGF mRNA and protein levels thereby contributing to the dysfunctional immune state in MF [19]. Treatment with CXCR1/2 inhibitor reduces levels of both IL8 and VEGF elaborated by co-cultured MF megakaryocytes with BM stromal cells suggesting downregulation of an autocrine feedback loop thereby eliminating one factor that contributes to MF immune dysfunction. More recently, Liu and colleagues have reported that IL-8 plays an important role in orchestrating tumor immunity in glioma patients, and that inhibition of IL-8/ CXCR1/2 potentiates immune checkpoint blockade efficacy [20] (Figure 1).
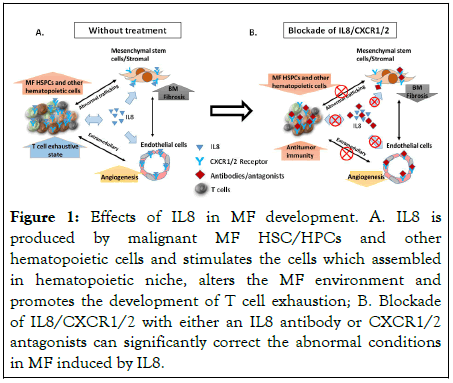
Figure 1:Effects of IL8 in MF development. A. IL8 is produced by malignant MF HSC/HPCs and other hematopoietic cells and stimulates the cells which assembled in hematopoietic niche, alters the MF environment and promotes the development of T cell exhaustion; B. Blockade of IL8/CXCR1/2 with either an IL8 antibody or CXCR1/2 antagonists can significantly correct the abnormal conditions in MF induced by IL8.
Strategies that interrupt the actions of IL8 on immunity should become a priority in the exploration of immune therapeutics for MF patients. The numerous biologic effects of IL8 provide a strong rationale for interrupting the IL8/CXCR1/2 axis with blocking antibodies, protein traps as well as receptor antagonists in treating MF patients. Imminently, a phase 1 clinical trial of reparixin will begin in patients with MF that attempts to interrupt the actions of IL8.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Hoffman R, Lu M (2023) The IL8 (CXCL8)/CXCR1/2 Axis: A Potential Target for the Treatment of Myelofibrosis. J Leuk. 11:328.
Received: 06-May-2023, Manuscript No. JLU-23-23931; Editor assigned: 08-May-2023, Pre QC No. JLU-23-23931 (PQ); Reviewed: 23-May-2023, QC No. JLU-23-23931; Revised: 31-May-2023, Manuscript No. JLU-23-23931 (R); Published: 07-Jun-2023 , DOI: 10.35248/2329-6925.23.11.328
Copyright: © 2023 Hoffman R, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.