Journal of Cancer Research and Immuno-Oncology
Open Access
ISSN: 2684-1266
+44-77-2385-9429
ISSN: 2684-1266
+44-77-2385-9429
Research - (2020)Volume 6, Issue 3
Background: Gastrointestinal stromal tumors (GISTs) are the most frequent tumors with mesenchymal origin at the level of the digestive tract. Assessment of intratumoral immune cells can provide valuable prognostic information and may contribute to the development of targeted immune therapies for selected cases. Here in we evaluated the inflammatory infiltrate in gastrointestinal stromal tumors, the ratios between cytotoxic and helper T cells and their prognostic significance.
Methods: We retrospectively analysed 25 cases of GISTs and extragastrointestinal stromal tumors (EGISTs). Immunohistochemical testing for CD3, CD4, CD8, CD20 and CD68 was performed to emphasize the immune cells. Inflammatory cells were quantified with the help of ImageJ software. Statistical analysis was performed to search for correlation between the immune response and clinical-pathological and prognostic variables.
Results: GISTs were in all cases infiltrated with immune cells in variable amount. The pattern of distribution was diffuse or in aggregates, most frequent around blood vessels. Gastric tumors had the largest amount of inflammatory infiltrate and EGISTs the lowest. The dominant intratumoral immune cells were represented by lymphocytes, with fewer plasma cells, histiocytes, eosinophils, neutrophils and mast cells. CD3+ lymphocytes were the most common subtype. In 9 cases the CD8+/CD4+ ratio was subunitary. An increased number of histiocytes were associated with a high risk of disease progression. No other correlation between immune cells and other prognostic factors were established.
Conclusion: Gastrointestinal stromal tumors represent the site of complex interactions between various types of immune cells and neoplastic cells. Accumulation of CD68+ cells correlates with high risk GISTs. Our paper provides an overview on the inflammation in this tumor type and further studies are necessary for more comprehensive results.
Gastrointestinal stromal tumor; Histopathology; Immunohistochemistry; Immune response; Inflammatory infiltrate; Lymphocytes; Macrophages
Gastrointestinal stromal tumors (GISTs) are the most common tumors with mesenchymal origin located in the digestive tract. They have a wide spectrum of biologic behaviour, and new approaches of prognostic evaluation and treatment are a matter of concern [1].
The immune system plays an essential role in tissue homeostasis, acting as a guardian, by initiating inflammatory responses in the presence of foreign or injurious stimuli. Tumor cells can also induce an immune response by altering the tissue structure. The complex interaction between immune and tumor cells was termed immunoediting and comprises three phases: elimination – in which the immune system annihilates tumoral cells, equilibrium – immune-mediated tumor dormancy and escape – when tumor cells are liberated of the immune suppression. Most of the patients are diagnosed in the escape phase [2-7].
Recent studies try to define inflammation and the inflammatory cells within or surrounding the tumor in the intent to determine their prognostic role, as evidence has shown that interconnections between them govern cancer evolution. Also, immune response can be regarded as a therapeutic target as in practice several immune treatments were adopted for malignant tumors and proved to prolong survival [8,9].
The microenvironment in gastrointestinal stromal tumors also contains immune infiltrates. Studies investigating the inflammation status in GISTs are very limited, but the findings are promising. Most of the inflammatory cells in GISTs are represented by macrophages and T cells [10]. B lymphocytes and natural killer cells are rare but in metastases seem to be more frequent. Due to the significant number of tumor-infiltrating immune cells, GISTs could be considered for immunotherapy, thus it is a territory that deserves to be exploited, especially considering the resistance to classical treatment manifested in a subset of cases [11]. As for factors that influence the immune response, Imatinib treatment, besides inhibiting tumor cells proliferation and survival, was proven to favourable impact the immune system, leading to activation of CD8+ cytotoxic T cells [12].
Gastrointestinal stromal tumors have a variable malignant potential influenced by the tumor dimensions, mitotic rate and location [13]. The role of the immune system in these tumors is far from being elucidated. In this paper we attempt to evaluate the immune infiltrate in gastrointestinal stromal tumors and establish its prognostic significance.
We ran a retrospective study on 25 cases of gastrointestinal stromal tumors diagnosed in our Pathology Department between 2016 and 2018, with the intention to describe the patterns of inflammation within the tumors, to quantify the immune infiltrate and also to immuno phenotype the immune cells.
For this purpose, we retrieved the archived pathology reports, medical records, pathology slides that were used for diagnosis and the afferent paraffin embedded tissue samples. We reviewed the existent hematoxylin and eosin (H and E) slides and the immunohistochemistry (IHC) slides that confirmed the diagnosis: CD117 (Rabbit Monoclonal Antibody, Clone YR145), DOG1 (Rabbit Monoclonal Antibody, Clone SP31), SMA (Mouse Monoclonal Antibody, Clone 1A4), S100 (Mouse Monoclonal Antibody, Clone 4C4.9), Ki67 (Rabbit Monoclonal Antibody, Clone SP6).
Also, new IHC tests were performed, to evaluate the immune cells. The markers used were represented by: CD3 (Rabbit Monoclonal Antibody, Clone MRQ-39) – a general marker for T lymphocytes, CD20 (Mouse Monoclonal Antibody, Clone L26) – a general marker for B lymphocytes, CD4 (Rabbit Monoclonal Antibody, Clone EP204)–to emphasise T helper cells, CD8 (Rabbit Monoclonal Antibody, Clone SP16) – for T cytotoxic cells and CD68 (Mouse Monoclonal Antibody, Clone Kp-1) – for macrophages. Positive expression meant membranous staining for CD3, CD4, CD8 and CD20 and cytoplasmic staining for CD68.
For quantifying the immune cells, 5 pictures of high power field (HPF) aspects from representative areas were taken per slide with the use of a CellSens program (Version 510_UMA_cellSens17- Indus-en_00) attached to a BX53 Olympus microscope. With the further use of ImageJ program, we determined the number of immune cells per HPF (40X). First, the pictures were converted in 8 bit variants, afterwards the threshold was manually adjusted, with visual control, until in the image were emphasised the structures of interest and finally, a count function was activated to display the number of items.
For the statistical analysis we used chi-square test using Microsoft Excel 2013 program, the results being considered significant for p values <0.05.
The patients involved in the research signed an informed consent, allowing the use of their tissues in scientific studies.
General clinicopathological features of gastrointestinal stromal tumors
25 tumors from a continuous, unselected cohort of patients with gastrointestinal stromal tumors were included in the study. Patient’s ages were between 28 and 73. 16 were females and 9 were males. 23 were primary tumors (19 with gastrointestinal location and 4 with extragastrointestinal location-EGISTs) and two were recurrences (one localised in the stomach and one in the rectum). The dimensions varied between 0.5 and 21 cm in greatest diameter.
For the histopathological diagnosis, between 4 and 19 H and E slides were evaluated for every case. 13 tumors showed spindle cell morphology, one was composed of epithelioid cells and 11 had mixed patterns. The mitotic rate was <5/50HPFs in 16 cases and >5/50HPFs in 9 cases. The diagnosis was confirmed immunohistochemically through positive staining for CD117 and DOG1. SMA and S100 were used for differential diagnosis. Also, a proliferation index (Ki67) was evaluated, varying between 2% and 50%.
Primary gastrointestinal stromal tumors (n=19) were classified in risk categories according to National Institutes of Health (NIH) risk stratification, that considers tumor dimensions and mitotic rate. Also, prognostic groups were assigned to them according to Armed Forces Institute of Pathology (AFIP), that supplementary considers tumor location [14,15]. GISTs characteristics and risk stratification are shown in Table 1.
| No. | Location | Dimension (cm) | Mitotic rate (per 50 HPFs) | Risk category (NIH) | Prognostic group (AFIP) |
|---|---|---|---|---|---|
| 1 | Gastric | 4.5 | <5 | Low | 2 |
| 2 | Retroperitoneum | 7 | >5 | - | - |
| 3 | Duodenum | 4.5 | <5 | Low | 2 |
| 4 | Gastric | 3.5 | <5 | Low | 2 |
| 5 | Gastric recurrence | 8.5 | <5 | - | - |
| 6 | Duodenum | 3.8 | <5 | Low | 2 |
| 7 | Jejunum | 8 | >5 | High | 6a |
| 8 | Gastric | 4 | <5 | Low | 2 |
| 9 | Ileum | 5 | <5 | Low | 2 |
| 10 | Retrovaginal | 21 | <5 | - | - |
| 11 | Omentum | 5 | <5 | - | - |
| 12 | Gastric | 20 | >5 | High | 6b |
| 13 | Rectum | 10 | >5 | High | 6a |
| 14 | Jejunum | 4 | >5 | High | 2 |
| 15 | Gastric | 3 | <5 | Low | 3b |
| 16 | Gastric | 7 | >5 | High | 6a |
| 17 | Gastric | 1.8 | <5 | Very low | 1 |
| 18 | Gastric | 3 | <5 | Low | 3a |
| 19 | Gastric | 8 | >5 | High | 6a |
| 20 | Gastric | 4.4 | <5 | Low | 2 |
| 21 | Gastric | 2.8 | <5 | Low | 2 |
| 22 | Peritoneal nodules | 3.5 | <5 | - | - |
| 23 | Rectal recurrence | 0.5 | <5 | - | - |
| 24 | Ileum | 12 | >5 | High | 6b |
| 25 | Gastric | 6.5 | >5 | High | 6a |
Table 1: GISTs characteristics and risk stratification.
Immune cells status for the studied cases
Intratumoral leukocytes were evaluated on a representative slide for each case. Necrosis and ulcerations were excluded from the analysis. On H and E stained slides, variable amounts of immune cells could be observed in each tumor. The pattern of distribution was either diffuses, with leukocytes scattered between tumor cells, either with focal accumulations, especially around vascular spaces or at the periphery of the tumor, or, in most instances, represented by a combination of patterns. The dominant cellular type was represented by lymphocytes, with occasional evident plasma cells, histiocytes, eosinophils, neutrophils or mast cells. Evaluation of the immune cells on H and E coloration had considerable limitations as these were difficult to identify and quantify, especially in hypercellular tumors, highly hyalinised tumors, tumors with a high mitotic rate or with numerous apoptotic bodies etc. Different patterns of inflammation (H and E, 40X) are shown in Figure 1.
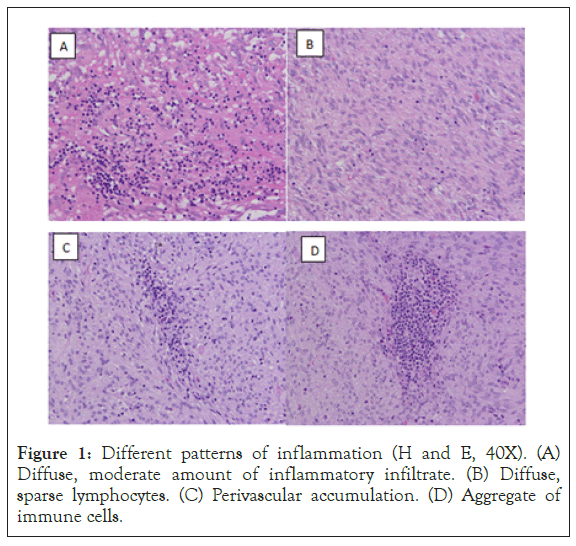
Figure 1: Different patterns of inflammation (H and E, 40X). (A) Diffuse, moderate amount of inflammatory infiltrate. (B) Diffuse, sparse lymphocytes. (C) Perivascular accumulation. (D) Aggregate of immune cells.
To further evaluate the inflammatory infiltrate from a qualitative point of view, immunohistochemistry was performed to mark T lymphocytes (CD3+) and their subtypes: helper (CD4+) and cytotoxic (CD8+), B lymphocytes (CD20+) and histiocytes (CD68+). In one case, the tumoral tissue was depleted in the course of processing, and it was excluded from the immunohistochemical analysis of the inflammation, remaining 24 cases.
For a quantitative analysis, to minimise the operator dependent bias determined by visual inspection, we took 5 photographs per slide and used a program specialised in image analysis (ImageJ) to count the number of marked cells. We counted the cells on the IHC slides, but also on the H and E slides. The amount of immune cells was expressed as number of cells per unit of surface (high power field) as shown in Figure 2.

Figure 2: The interface and the work mode in the ImageJ program.
Resulted that GISTs are infiltrated, with no exception by immune cells, in variable number, the dominant immuno type being represented by CD3+ lymphocytes (Table 2, Figures 3 and 4).
| H and E | CD3+ | CD4+ | CD8+ | CD20+ | CD68+ | |
|---|---|---|---|---|---|---|
| Range | 5-776 | 0-324 | 0-241 | 0-222 | 0-470 | 0-310 |
| Median | 81 | 63 | 48 | 36 | 24 | 37 |
| Mean | 102 | 90 | 48 | 97 | 77 | 44 |
| Mode | 73 | 95 | 0 | 34 | 0 | - |
| STDEV | 88 | 53 | 42 | 40 | 53 | 31 |
Table 2: The amount of immune cells (number of cells per high power field).
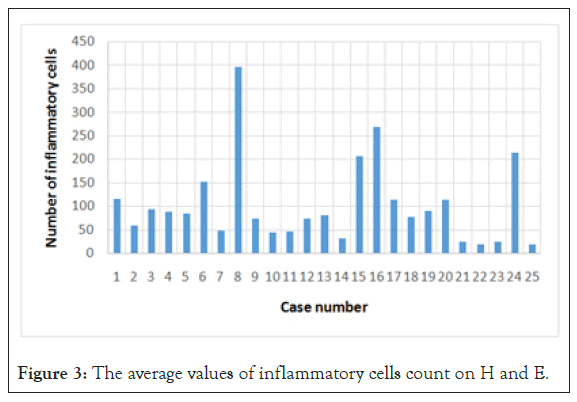
Figure 3: The average values of inflammatory cells count on H and E.
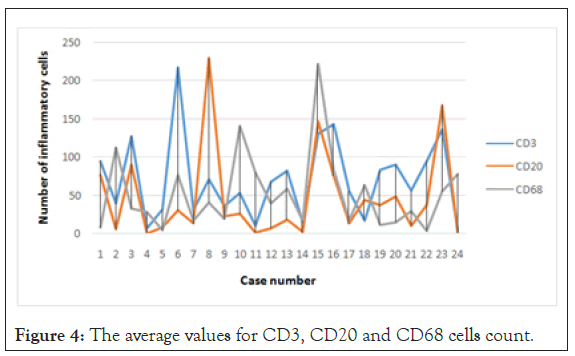
Figure 4: The average values for CD3, CD20 and CD68 cells count.
The average number of immune cells counted on H and E slides was the greatest in gastric tumors and the lowest in EGISTs shown in Table 3.
| Gastric | Small bowel | EGIST | Recurrence |
|---|---|---|---|
| 131 | 102 | 42 | 55 |
Table 3: Average number of immune cells (counted on H and E) on different locations.
The age of the patients showed no correlation with the amount of immune cells (p=0.44, CHITEST).
We calculated the ratios between cytotoxic T cells (CD8+) and helper T cells (CD4+). In 13 cases the ratio was greater than 1 and in 9 cases it was lesser than one. In search for an association between CD8/CD4 ratio and the risk of disease progression according to NIH criteria, we verified if a sub unitary ratio appears more frequently in high risk tumors. The p value of the Chi square test was a marginal value equal to 0.056. Instead, a higher number of CD68+ cells was associated more frequently with a high risk GIST (p=0.035).
No correlation was observed between the number of CD20 lymphocytes and the Ki 67 value (p=0.22).
Immune cells play an important role in oncogenesis, tumor progression and response to treatment, its prognostic role being investigated by an increasing number of scholars. Multiple types of immune cells were described to be present in the tumor microenvironment such as lymphocytes, macrophages, neutrophils, dendritic cells, natural killer cells [16-18].
T lymphocytes are a diversified group of leucocytes characterised by the expression of TCR and CD3, among other specific molecules. They can be divided in two classes, based on the surface molecular expression: CD8+ -cytotoxic T cells, capable of recognising and neutralizing malignant cells and CD4+-helper T cells that secrete immune modulators, influencing other cells responses [19]. Numerous studies have shown that a high level of intratumoral CD3+ T cells represents a favourable prognostic factor in tumors such as melanoma, ovarian cancer, head and neck, breast, colorectum, lung cancers etc [20-25]. In GISTs, CD3+ cells proved to be the most frequent lymphocytes, more abundant in metastases than in primary tumors and in small bowel and colon, compared to the gastric location. Also, in cases with a proliferation index >10%, the number of CD3+ lymphocytes was higher than in the cases with Ki67<10% [11,26]. In our study, CD3+ lymphocytes outnumbered other classes of immune cells but no correlations with diverse clinical-pathological variables could be demonstrated. CD8+ T effector cells, which release cytotoxins, were proven to improve survival in colorectal carcinoma but were accompanied by a dismal prognosis in anal squamous carcinoma [27,28]. In gastrointestinal stromal tumors, imatinib therapy was demonstrated to stimulate CD8+ cells activity [11]. Some studies claim that the CD8/CD4 ratio has a better predictive value than the independent values of the two [29,30]. We also calculated the CD8/CD4 ratios in the attempt to discover if tumors with a ratio<1 are associated with a high risk of disease progression and we obtained a value of p of 0.056, meaning that, for an accurate conclusion we need to extend our study in the future, on a larger cohort of patients. Other studies show that the presence of CD4+ Th1 response proved to be a positive prognostic marker in breast carcinoma, medulloblastoma, gastric cancer etc and a Th2 demonstrated no prognostic value [16].
B lymphocytes are characterized by the expression of a specific B cell receptor (BCR) and of other surface markers among which CD20 is routinely used for identifying this cell type. They recognise antigens, present them to CD4+ T cells, the latter producing cytokines that can stimulate the expansion and maturation of B cells in antibodysecreting plasma cells or memory B cells [31]. Increased amounts of CD20+ B cells were associated with an improved clinical evolution in tumors such as hepatocellular carcinoma, melanoma, prostate carcinoma etc. [32-34]. In gastrointestinal stromal tumors, B cells were more numerous in cases with a higher Ki 67 index [35]. In our study, intratumoral CD20+ cells were well represented but no correlation was observed with the proliferation index.
Macrophages are characterised by the expression of the cell surface markers including, but not limited to CD68. Studies that tried to correlate intratumoral macrophages with the disease outcome had extremely heterogeneous results. An unfavourable clinical course was demonstrated in breast carcinoma, lung adenocarcinoma, melanoma etc., associated with a high number of CD68+ cells. In other studies an improved clinical outcome was demonstrated, as in gastric cancer, non-small cell lung carcinoma, hepatocellular carcinoma etc. [16]. In gastrointestinal stromal tumors, the quantity of macrophages does not correlate with prognosis or treatment. Also, metastatic GISTs proved to have twice as much intratumoral macrophages as primary GISTs, suggesting their role in tumor progression [10]. The tumors in our study proved to associate large number of macrophages with a high risk of disease progression.
In recent years multiple attempts to standardize the evaluation of tumor associated immune response have emerged. Immunoscore was one of the proposals. This is based on determining the quantity of two different lymphocytic populations from CD3, CD8 and CD45RO, in the center of the tumor and at the invasive margins. The result is a score from 0 to 4 (a higher score reflecting a higher density of immune cells in the two compartments). For colorectal carcinoma, the only instance where it has been validated, a higher amount of immune cells correlated with increased survival. We didn’t find Immunoscore suitable in our study as GISTs are rather characterised by pushing borders, not by actual invasive margins [36].
Another proposed method, described initially on breast cancer and subsequently tested on other tumor entities is based on determining the quantity of T lymphocytes, as percentage of section area, among the tumor cells and also in the tumoral stroma, inside the tumor borders. No thresholds were established in this case to separate different prognostic groups. This method was not applicable in our study, because the intratumoral stroma cannot be distinguished from the tumor cells in most of the GISTs [37,38].
Quantification of immune infiltrate in GISTs was facilitated by ImageJ software that proved to be a very useful tool for counting cells. Nevertheless, it has a series of limitations. It recognises the pixels and not only the elements of interest have the same range of pixels but also some artefacts, mitoses, tumor cells nuclei etc. But this drawback is in most part correctable by a careful visual control accompanied by a proper adjustment of the counting threshold so that as few as possible alien elements are registered. Therefore, this image analysis software turned out to be a very helpful instrument that made possible an accurate quantitative examination of the intratumoral immune cells, and this is certainly superior to visual counting.
Gastrointestinal stromal tumors are richly infiltrated by inflammatory cells, the dominant immune cell type being represented by T lymphocytes. An increased number of intratumoral macrophages were associated with high risk GISTs. Due to the limited number of cases no other correlations could be established between the type and amount of inflammatory cells and prognostic parameters, but more extensive future studies could improve the understanding of the impact of immune cells on the evolution of gastrointestinal stromal tumors.
Citation: Herlea V, Rosulescu A, Iorgescu A, Dima SO, Dumitrascu T, Brasoveanu V, et al. (2020) The Immune Response in Gastrointestinal Stromal Tumors. J Cancer Res Immunooncol. 6:125.
Received: 08-Sep-2020 Accepted: 22-Sep-2020 Published: 29-Sep-2020 , DOI: 10.35248/2684-1266.20.6.125
Copyright: © 2020 Herlea V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.