Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Review Article - (2022)
For long, germline mosaicism has been considered a very rare event whereas it was in fact underestimated because of its lack of diagnosis. Thanks to the development of NGS (next generation sequencing) applied to intergeneration studies; its occurrence is now better diagnosed. Genomic variations characteristic of this phenomenon can be either Single Nucleotide Polymorphism (SNP) or Copy Number Variations (CNV). De novo mutations resulting in germline mosaicism can appear either pre or postzygotically. If the mutation occurs postzygotically, the mutation may exist in the germ cells but also in the soma. In that case, the mosaic carrier is healthy because of the low level of expression of the mutation. However, the mutation will be transmitted to all cells of the offspring that will express the disease. On the contrary, if the mutational event occurs prezygotically in the germ cells than there is no expression in the soma. The risk of transmission to the offspring will rely on the percentage of germ cells carrying the deletion. In particular, in the paternal germline, it has been shown that this risk increases with paternal age and is referred as paternal age effect. Indeed, the Spermatogonial Stem Cell (SSC) pool is maintained through a high rate of cell divisions throughout a men’s lifespan, with hence a higher risk of errors from mitosis as the individual ages. In some cases, the acquired mutation gives a growth advantage to SSCs, leading to the clonal expansion of mutated cells in the testis and thereby increasing the number of spermatozoa carrying the pathogenic mutations. In that case, the best diagnosis would be to perform sperm analysis to ascertain the diagnosis and to evaluate the accurate risks of transmission. Therefore, there is a necessary need to develop routine diagnosis on sperm to search for germline mosaicism.
Germline mosaicism; Next Generation sequencing; Paternal age effect
Mutations generate sequence diversity and provide a substrate for selection. It is estimated that the de novo rate of Single Nucleotide Variants (SNVs) is approximately 10-8 per generation [1]. During the fertilization process, a human zygote inherits half of its genetic content from his father and his mother. However, during this process, there are some genetic changes, called De Novo Mutations (DNMs) that occur either pre or post-the zygote stage, allowing each individual to be unique. It is estimated that at least 50-100 new point mutations arise in one individual’s genome compared to their parent genomes. Those DNMs can have no effect on one individual’s health but can sometimes be deleterious and give rise to a range of disease [2]. Mutation is considered de novo if it cannot be found in the parent’s blood cells, hence when a first child is born with a disease no more explorations are conducted and the estimated risk of recurrence is very low (1%). However, the recurrence of the same genomic variation in a sibling must evoke germline mosaicism in one parent [1]. There are different genomic variations that can explain germline mosaicism; it can be Single Nucleotide Polymorphisms (SNPs), Copy Number Variations (CNVs) or aneuploidies. The occurrence of each variation depends on its stage of appearance. Aneuploidies are more frequent in the first stages of embryo development whereas SNPs mostly occur in the paternal germline and increase with paternal age [3].
Different germline mosaicism mechanism
Postzygotic mosaicism (PZM): During the early postzygotic development of the embryo, a DNM event can happen allowing a variant to segregate in one or some tissues of the individual, generating a mosaic state for this specific mutation. This event is identified as a Postzygotic Mosaicism (PZM), also called gonosomal mosaicism. However, considering the distribution of the mutation during embryo division, it can be either limited to the soma, and the children carrying the mosaicism will be designed as a child with a postzygotic mosaicism (child-PZM, second generation), whereas in some situations, the mosaic can be inherited in both the soma and the germ cells. The mutation is then carried by the gametes of the second generation individual (parent-PZM) and can be transmitted to the third generation [4] (Figure 1). Depending on the expression level of the DNM and its tissue expression the individual can be symptomatic or asymptomatic. When a first child is born with a symptomatic phenotype, the difficulty is to identify the mosaicism, particularly when the expression level is low and limited to few cell types. In this case, the mutation can be wrongly considered as constitutive and the risk of recurrence in a sibling mistakenly estimated very low. Indeed, if the DNM is also present in the germ cells, there is a high risk of recurrence in a sibling [2]. The particularity of the parent-PZM transmission mode is that it can be transmitted either from the mother or the father gamete and is independent of the parent’s age. Similarly, no significant enrichment of particular gonosomal mutation types has been observed [5].
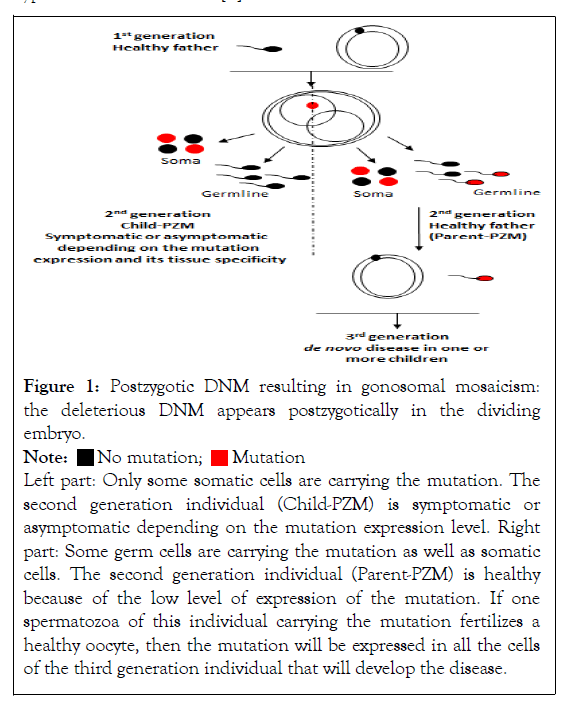
Figure 1: Postzygotic DNM resulting in gonosomal mosaicism: the deleterious DNM appears postzygotically in the dividing embryo.
Left part: Only some somatic cells are carrying the mutation. The second generation individual (Child-PZM) is symptomatic or asymptomatic depending on the mutation expression level. Right part: Some germ cells are carrying the mutation as well as somatic cells. The second generation individual (Parent-PZM) is healthy because of the low level of expression of the mutation. If one spermatozoa of this individual carrying the mutation fertilizes a healthy oocyte, then the mutation will be expressed in all the cells of the third generation individual that will develop the disease.
Prezygotic mosaicism: On the other hand, there are some DNMs described as prezygotic mutations that arise in the parental germline before fertilization. In this case, the mutation takes place in the germline and is absent from the blood or any other tissue of the individual. Even this phenomenon can happen either in the paternal or maternal germline, it is intimately linked to DNA replication, explaining why this mechanism takes place three to four times more often in the paternal germline, where many more germline cells divisions happen [4], than in the maternal germline [6,7]. Indeed, the Spermatogonial Stem Cell (SSC) pool is maintained through a high rate of cell divisions, with hence a risk of errors from mitosis as the individual ages, while the oogonial stem cell pool is established before birth. The germline mosaicism described as arising with paternal age is referred to the Paternal Age Effect (PAE). More particularly, a subset of DNMs have been described in the genes encoding components of the tyrosine kinase receptor/RAS-MAPK pathway and are responsive of a range of developmental disorders such as Noonan, Costello and Apert syndromes, achondroplasia…Those disease are more frequently observed in the children of older fathers. The highly recurrence of mutations in this pathway can be explained by the fact that they give a growth advantage leading to the clonal expansion of mutated cells in the testis and thereby increasing the number of spermatozoa carrying the pathogenic mutations (Figure 2a and 2b). As the mutant spermatogonial clone increases over cell divisions, it results in a higher risk of transmission with paternal age [8]. They are referred as selfish mutations, showing an extreme paternal bias in origin and in this case, are more specifically designed as selfish spermatogonial selection [9]. This process is identical to oncogenic driver mutations observed in tumors. The hypothesis of a paternal germline specific mutation has been considered because of the higher spontaneous birth rate of children carrying those pathologies associated with delayed paternity. It can be expected that some of the DNMs taking place in the SSCs are ~1000-fold more frequent than the expected background mutation rate [8].
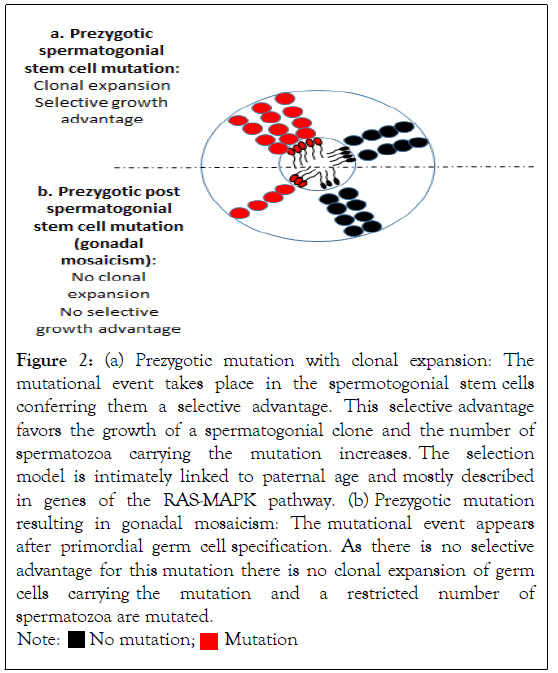
Figure 2: (a) Prezygotic mutation with clonal expansion: The mutational event takes place in the spermotogonial stem cells conferring them a selective advantage. This selective advantage favors the growth of a spermatogonial clone and the number of spermatozoa carrying the mutation increases. The selection model is intimately linked to paternal age and mostly described in genes of the RAS-MAPK pathway. (b) Prezygotic mutation resulting in gonadal mosaicism: The mutational event appears after primordial germ cell specification. As there is no selective advantage for this mutation there is no clonal expansion of germ
cells carrying the mutation and a restricted number of spermatozoa are mutated.
However, a third type of germline mosaicism has been described. In this case, the mutation also appears prezygotically but after primordial germ cell specification (Figure 2b). It is called post-primordial germ cell mosaicism or gonadal mosaicism. In this case, the acquired mutation does not give a selective advantage to the germ cells carrying the deletion, contrarily to the selfish selection mechanism described above. Thereby, there is no clonal expansion of the cells carrying the mutation which remains restricted to a small pool of germ cells. In case of a gonadal mosaicism, there is no bias to a paternal effect and no correlation with the age of the parent [5,10].
Diagnosis of germline mutations
For long, germline mosaicism has been considered a very rare event because most of the time the diagnosis of mosaicism could not be done. However, with the improvement of genetic analysis technologies applied to multiple generation family studies, it has been estimated that ~3% of germline DNM originated as mosaic in the germ cells of a parent and that nearly 10% were postzygotic both present in somatic and germ cells [5].
Genome and exome sequencing analysis on blood DNA
Family pedigree studies using the Next Generation Sequencing (NGS) are an informative way to analyse germline mosaicism. NGS, as standard Whole Genome Sequencing (WGS) and ultrahigh-depth sequencing technologies, is a sensitive genotyping technique that can detect very low-level mosaicism in a tiny fraction of cells that would not be identified using classical Sanger sequencing method or microarray-based techniques. This is particularly relevant when the variant is expressed at very low levels in blood cells or saliva, as they are the most frequently available samples for genetic analysis [4]. Wright, et al. have used trio-whole exome sequencing on blood or saliva samples of parent-offspring families. This sequencing technology enabled them to differentiate post zygotic mosaicism carried by a child only in its somatic cells (Child-PZM) from post zygotic mosaicism carried by a unaffected parent (parent-PZM) both in his somatic and germ cells. They could confirm that the recurrence risk in future siblings is negligible if a postzygotic mosaicism is diagnosed in the first child (child-PZM), corresponding to an isolated event that happened during embryo divisions, whereas this risk could reach up to 50% when inherited from a parental gamete (parent-PZM) carrying the mutation [4]. Sasani, et al. have conducted their analysis on three-generation human families. This analysis has allowed them to differentiate the mutations occurring pre and postzygotically by studying its origin of transmission (maternal or paternal) and the existence of a bias towards paternal age. The principle is to conduct whole DNA sequencing from blood samples of several individuals belonging to the same family to identify DNMs in the second and third generation. They could trace and differentiate gonosomal mosaicism from pre and post primordial germline mutations by studying its rates of transmission in the second generation and the third generation.
Using this screening on 603 individuals from 33 families, they could identify 4671 germline DNMs in 70 second generation’s individuals, most of them being SNVs (92%). They could estimate the rate of germline mutations in the second generation around 1.1 x 10-8 per base per generation for SNVs which corresponds to an average number of 70 de novo SNVs per genome. Studying third generation DNMs, they could estimate that 21% of them were originating from a parental gamete of origin with a marked participation of the male gametes (78.7% transmitted from the father) [5].
Direct gamete analysis of the mosaic mutation
It is possible to look directly for the mutation in the gametes responsible for the birth of an affected child. While this analysis is mostly impossible in the female germline, considering the inaccessibility of the genetic material to conduct the analysis, sperm is an easy access biological sample containing millions of spermatozoa to analyse. However, in this case, only a specific DNA region can be studied targeting preselected mutations. Very sensitive sequencing technologies can be used as highthroughput sequencing or duplex sequencing but also droplet digital PCR allowing ultra-rare variant detection. Salazar, et al. used the duplex sequencing technology to screen the coding region of the FGFR3 gene implicated in achondroplasia. They could identify a higher incidence of mutations in the sperm of older donors confirming the clonal expansion hypothesis of mutated SSCs in the testes of older men [11]. Frisk, et al. performed an analysis of 31 sperm samples of fathers with affected children by droplet digital PCR in parallel of WGS blood analysis. In one sperm, they could identify a causal mutation that was not present in the blood, confirming prezygotic mosaicism, whereas in one other individual mosaicism was detected both in blood and sperm samples, confirming post zygotic mosaicism [7]. Similarly, Breuss, et al. conducted WGS in parallel on height sperm and blood samples. They could identify 34.8% of SNV in sperm only, 56.5% in blood and sperm and 8.7% in blood only [10].
FISH on spermatozoa can be used for CNV detection, as we did in a recent case report of a sperm donor carrying a deletion in the 7q32. 1q33 region. In this case, a specific probe was designed after identifying the deletion by Array-CGH in the amniotic fluid of the affected fetus [3].
Identification of the mutate SSC clones in the testis
The hypothesis of a clonal expansion of SSCs carrying a DNM has been built on the higher frequency of affected children as their father ages. However, to confirm this hypothesis, direct analyses on testicular tissue have been conducted. Spatial distribution analyses of a targeted mutation have been conducted directly on the testicular tissue to identify clusters of mutated SSCs. For this analysis, testicular tissue has been cut in small pieces and a DNA extraction has been conducted in each piece. Mutation frequency analysis has been realized through a quantitative detection mutation assay. Using this methodology, Qin, et al. were able to identify “hot” pieces of testicular samples, that compared to other pieces were carrying a very high mutation frequency for the C755G mutations encountered in the Apart syndrome [12]. Maher, et al. also conducted mutations hotspot analysis on micro dissected tubules extracted from the testicular biopsies of fourteen individuals. They were able to identify several mutations enriched for clonal events promoted by positive selection of mutant sperm cells [13].
Immunostaining tubules analysis
Lim, et al. have analyzed, by immunostaining, serial sections of formalin fixed paraffin embedded testes using antibodies against targeted genes of the RAS-MAPK pathway [14].They identified immunopositive tubules containing dense clusters of spermatogonia positive for the mutations.
These observations confirmed that some mutations are highly clustered inside some seminiferous tubules and the selfish spermatogonial selection process. Of course, those analyses cannot be done routinely, as they need a direct access to testicular tissue.
In conclusion, the diagnosis of germline mosaicism is not easy. The birth of a first child with a DNM does not usually recall for a risk of germinal mosaicism and it is usually when a second child is carrying the same mutation that this hypothesis is studied. Moreover, even new sensitive genotyping technologies have been developed to detect low-level mosaicism in blood samples; they are not still routinely used. Furthermore, the risk of transmission to the descendants is difficult to estimate as it depends on the proportion of mutated germ cells and their resulting spermatozoa. To estimate this risk, routine diagnosis to search for germline mosaicism have to be developed not only on blood but also on sperm when a paternal transmission has been established.
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
[CrossRef] [Google Scholar] [Pubmed]
Citation: Chalas C, Patrat C (2022) The Importance of Germline Mosaicism in the Occurrence of De Novo Mutational Events and its Necessary Diagnosis.Andrology. S2:003.
Received: 22-Apr-2022, Manuscript No. ANO-22-17117; Editor assigned: 28-Apr-2022, Pre QC No. ANO-22-17117 (PQ); Reviewed: 20-May-2022, QC No. ANO-22-17117; Revised: 30-May-2022, Manuscript No. ANO-22-17117 (R); Published: 07-Jun-2022 , DOI: 10.35248/2167-0250.22.S2.003
Copyright: © 2022 Chalas C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : NO