Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2017) Volume 8, Issue 1
The thermally stable trans isomers of donor-acceptor substituted azobenzene dyes can be easily transformed into unstable cis-form by light. Although azobenzene and its derivatives have been extensively investigated over the years, many aspects of the isomerization reaction kinetics are still unclear or controversial at best. Here, we investigate the kinetics of the thermal cis-trans isomerization of 4-anilino-4'-nitroazobenzene (4A4NAB) in six solvents of different polarities by means of flash photolysis. Using the transition state theory, we were able to determine a number of thermodynamic quantities including the standard enthalpy of activation Δ‡H0, the standard entropy of activation Δ‡S0, the Gibbs free energy of activation Δ‡G0, and the quasi-equilibrium constant K‡ of the isomerization reaction. Higher rates of isomerization and lower activation energies were observed in polar media, consistent with the formation of a charged transition state complex and the presence of solute−solvent interactions such as intermolecular hydrogen bonding. The data suggest that the cis-trans thermal isomerization of 4A4NAB is highly influenced by solvent polarity and the isomerization mechanism is likely to proceed via rotation about the azo double bond.
Keywords: Isomerization; Photo-isomerization; Electron-donor; Cis-trans
Azobenzenes are common organic dyes that have been extensively studied both experimentally and theoretically owing to their potential applications in material science, medicinal chemistry, molecular switches and other devices [1-15]. They are photo-reactive molecules that undergo reversible photo-isomerization from the more stable trans -isomer to the less stable cis-isomer. Despite decades of research, the mechanism of the cis-trans isomerization is not unequivocally established and depends largely on the experimental conditions (i.e., solvent polarity and viscosity), as well as molecular substitutions on the basic azobenzene molecule (i.e., azobenzene derivatives), both of which have a dramatic effect on the spectroscopic properties of the molecule and the kinetics of isomerization.
Photo-isomerization mechanisms
The 4-Anilino-4'-nitroazobenzene (4A4NAB) is a “push-pull” type molecule that can undergo a resonance structure due to the presence of an electron-donor (the anilino group) and an electron-acceptor (the nitro group) at the p and p' position of the conjugated aromatic system in the same molecule (Figure 1).
Two possible mechanisms have been proposed for the reversible photo-isomerization of azobenzenes. The inversion mechanism is suggested to proceed via a linear transition state in which the N=N double bond remains intact, whereas the rotation mechanism is proposed to occur via a twisted transition state in which the N=N π-bond is broken. Upon photo-excitation of the trans form, an electron is excited from its ground-state (S0) orbital to its first singlet excited state (S1) or second singlet excited state (S2) in which the electron retains its spin under an n-π* excitation condition or a π-π* excitation condition, respectively [1,2]. Azo groups are reported to photo-isomerize via two distinct mechanisms: the π-π* transition with an out-of-plane rotation mechanism in which the nitrogen-nitrogen π bond is ruptured heterolytically and a dipolar transition state is involved (Scheme 1), or the n-π* electronic transition with an inversion of one sp2 hybridized nitrogen atom through an sp hybridized linear transition state in which the double bond is retained (Scheme 2) [2-6]. The rate of isomerization for the inversion mechanism is relatively rapid and mostly independent of the polarity of the medium or the electronic nature of substituents on the azobenzene, but the rate for the rotation mechanism increases rapidly with increasing solvent polarity [3].
Scheme 1: Rotation mechanism for the dark cis-to-trans isomerization reaction of azobenzene following a photo-conversion of the trans -stable isomer to the cis-unstable isomer. Note that the electronic transitions discussed above pertain to the trans to cis reaction and not the cis to trans reaction.
A conceptual diagram of the reaction coordinate for the cis-trans isomerization of disperse orange 1 is shown in Figure 2 whereby the absorption of a photon of visible light causes the promotion of the stable ground-state form (trans-disperse orange 1) into an electronically excited state. The excited molecule quickly relaxes back to the ground electronic state of the cis-form. The ground-state cis molecule will then convert back to the trans isomer with the activation energy for the isomerization process shown as Ea in Figure 2.
Taking advantage of the unique properties of azobenzenes, this study explores important aspects of photochemistry and the effect of six different solvents (cyclohexane, toluene, benzene, tetrahydrofuran, acetone, and 3-pentanol) of different polarity on the kinetics of the cistrans isomerization of 4-anilino-4’-nitroazobenzene using a camera flash and a UV/Vis spectrophotometer. The camera flash initiates a photochemical isomerization of 4A4NAB (trans → cis) in which the stable trans -isomer is initially promoted to an electronically excited species (step 1 in Figure 2) followed by a decay to the ground-state cis isomer of lower energy level (step 2 in Figure 2). Subsequently, the cis isomer undergoes a thermal isomerization to the more stable trans isomer (step 3 in Figure 2). The trans → cis → trans isomerization process is completely reversible and could be carried for several cycles. Due to the steric hindrance of the two phenyl rings, the cis isomer decays down to the more energetically favored trans isomer (Figure 2). In this experiment the absorbance of a disperse orange 1 solution will be monitored at a wavelength where only the trans isomer absorbs. Upon exposure to the camera flash, a certain portion of the trans molecules will be converted to the cis isomer and the absorbance will drop before it starts to rise back to its original value as the cis molecules convert back to the trans isomer. This absorbance value is labeled A∞ (the absorbance at infinite time). A typical experimental scan of a flash photolysis experiment is shown in Figure 3.
Integrated rate law and the transition state theory thermodynamic equations
The overall isomerization reaction can be written according to the following scheme:
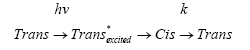
The rate of the thermal reaction Cis → trans can be written as:
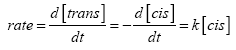 (1)
(1)
where k is the first order rate constant, and [trans] and [cis] are the concentrations of the cis and trans isomers, respectively at any time t. Knowing that the total number of molecules is constant and assuming that at t=∞ all the molecules have converted back to the trans isomer gives:
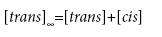 (2)
(2)
so that
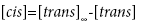 (3)
(3)
Substituting equation (3) into equation (1) yields:
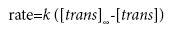 (4)
(4)
which upon integration gives:
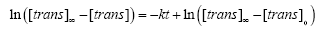 (5)
(5)
where [trans]0 is the concentration of the trans isomer at time t=0. Because the concentration of the trans isomer at any time t is proportional to the absorbance of the solution (via. Beer's Lambert law), the integrated rate law may then be written as:
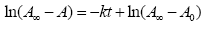 (6)
(6)
where A, A0, and A∞ are the absorbances at time t, t0, and t∞, respectively.
The dependence of the rate constants on temperature can be used to determine the reaction’s activation energy and the Arrhenius constant A according to Arrhenius equation 7:
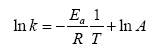 (7)
(7)
Using the transition state theory, the activation energy Ea and Arrhenius constant A can be used to determine the standard enthalpy of activation Δ‡H0, the standard entropy of activation Δ‡S0, the Gibbs free energy of activation Δ‡G0, and the quasi-equilibrium constant K‡ using the following equations:
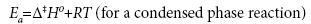 (8)
(8)
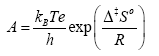 (9)
(9)
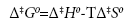 (10)
(10)
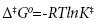 (11)
(11)
where kB is the Boltzmann constant, e is the Euler number 2.718…, and h is Planck’s constant.
All solvents employed in this study were used without further purification. The dye, 4-anilino-4’-nitroazobenzene was purchased from MP Biomedicals Inc. under the common name disperse orange 1, and is typically 15-25% by mass 4-anilino-4’-nitroazobenzene. There was no detailed safety information available on the manufacturer’s website other than to wear suitable protective clothing, gloves and eye/ face protection. In order to minimize degradation, the dye was freshly prepared in the appropriate solvent using a pinch of the red coloured dye and the stock solution was either wrapped in aluminium foil or stored in an amber bottle away from direct light. Any salts that did not dissolve in the organic solvents were decanted before the dye solutions, whose absorbance values ranged between 0.4 and 0.5, were used. In case of higher absorbance values, the dye solutions were appropriately diluted in the corresponding solvent. The kinetic data were essentially identical using a freshly prepared dye solution or one that was stored overnight in the dark for complete equilibration. However, it is important to keep all solutions tightly sealed to prevent evaporation.
The source of the solvents used in this study and their purity are as follow: cyclohexane (Acros Organics), ACS Grade, >99% (GC grade); toluene (Fischer Scientific), ACS certified, >99.5%; benzene (Mallinckrodt Pharmaceuticals), ACS Certified; tetrahydrofurane (EMD Millipore Corp.), ACS/USP Grade, >99.9%; acetone (Pharmco- Aaper), ACS/USP grade, >99.9%; 3-pentanol (Sigma-Aldrich), ≥ 98% (GC grade). A Varian Cary 50 Bio UV-Visible spectrophotometer with a Northwest TC 125 Quantum temperature control unit (ΔT ± 0.1°C) were used. The excitation light flash was produced by a Quantaray MS-1 camera flash.
The solvent-dependent maximum wavelength at which the trans isomer π* ← π transition occurs is shown in Table 1. Once the required temperature is reached, the sample was quickly removed from the spectrometer cell holder and flashed with the camera flash (by placing the flash against the quartz cuvette and depressing the flash button) and then immediately placed back into the spectrometer. The sample absorbance was monitored over time until its initial absorbance value before excitation was reached.
| Solvent | λ/nm | Relative Polarity | 1st order Rate Constant (k×103/s-1) | Log(k) |
|---|---|---|---|---|
| Cyclohexane | 431 | 0.006 | 1.38 ± 0.09 | - 2.86 ± 0.07 |
| Toluene | 445 | 0.099 | 1.89 ± 0.08 | - 2.72 ± 0.04 |
| Benzene | 445 | 0.111 | 1.87 ± 0.05 | - 2.73 ± 0.03 |
| THF | 463 | 0.207 | 25.9 ± 0.8 | - 1.38 ± 0.02 |
| Acetone | 468 | 0.355 | 95.5 ± 4.1 | - 1.02 ± 0.04 |
| 3-Pentanol | 485 | 0.463 | 449.2 ± 2.8 | - 0.35 ± 0.01 |
Table 1: First-order rate constants for the cis-trans isomerization of 4A4NAB at 25°C in six different solvents. The relative polarity and maximum wavelength of each solvent are also listed. The reported uncertainties are standard errors determined from replicate measurements.
The absorbance spectra of the 4A4NAB solutions in six different solvents are shown in Figure 4. The data show a bathochromic shift in 3-pentanol compared to cyclohexane. This pronounced red-shift of ~60 nm can be rationalized by hydrogen bond formation between the solute and the solvent and a more stable zwitterionic form of the dye in the more polar solvents. Additionally, the polarity of the solvent may affect the N-N-C angle and the energy of electronic transitions of the N=N bond [16,17].
Figure 5 shows the typical absorbance change vs. time when a 4A4NAB dye solution is excited with a camera flash. Before excitation, the steady absorbance indicates a stable solution (mostly molecules in the trans -form) at equilibrium. Once excited, a sudden drop in absorbance is observed followed by a return to the initial values as the 4A4NAB molecules transition from the unstable cis orientation to the more stable trans orientation. For each solvent, the experiment was repeated two to three times to ensure reproducibility, and at each temperature the same dye solution was excited/ flashed two to three times.
The rate constants for the isomerization of 4A4NAB in cyclohexane, toluene, benzene, tetrahydrofurane, acetone and 3-pentanol are determined from a plot of ln(A∞-A) vs. time as shown in Figure 6. In each case, the reaction followed the expected first order kinetics with the slope of the line equals to k. Clearly, the results of Figure 6 show a pronounced effect of solvent polarity on the rate of isomerization.
Table 1 summarizes the kinetics data obtained with six solvents whose relative polarity values are defined by the solvent polarity parameter as proposed by Reichardt [18]. The data in Table 1 show that the rate constants for the isomerization reactions increase significantly with an increase in the solvent relative polarity.
A plot of log(k) vs. relative polarity (Figure 7) exhibits a linear relationship consistent with earlier literature data for similar isomerization reactions involving other azobenzenes [18-22]. The increase in the rate constants of isomerization is accompanied by a decrease in activation energy which is typically the case with paradonor, para-acceptor type azobenzenes [21,22].
To determine the activation energy of the reaction, a plot of the natural logarithm of the isomerization rate versus temperature was created (Figure 8). The slopes and Y-intercepts of these plots represent the activation energy E‡ a and the Arrhenius frequency factor A for the isomerization reactions, respectively. All other thermodynamic parameters including the standard enthalpy of activation Δ‡H0, the standard entropy of activation Δ‡S0, the Gibbs free energy of activation Δ‡G0, and the quasi-equilibrium constant K‡ for the cis-trans isomerization process are summarized in Table 2.
| Solvent | RelativePolarity | Isomerization Energy (E‡a×10-3mol/J) |
Arrhenius Constant, Ln(A) |
Enthalpy of isomerization (Δ‡H0× 10-3mol/J) |
Entropy of isomerization (Δ‡S0/K.mol/J) |
Gibbs Free Energy Δ‡G0× 10-3 mol/J) |
Equilibrium Constant K‡ |
|---|---|---|---|---|---|---|---|
| Cyclohexane | 0.006 | 72.7 ± 0.2 | 22.7 ± 0.7 | 70.2 ±1.9 | - 64.0 ± 6.3 | 89.3 ± 2.7 | (2.26 ± 0.07) ´10-16 |
| Toluene | 0.099 | 76.3 ± 1.1 | 24.6 ± 0.4 | 73.8 ±1.1 | 49.5 ± 3.5 | 88.5 ± 1.5 | (3.01 ± 0.05) ´10-16 |
| Benzene | 0.111 | 71.0 ± 1.5 | 22.4 ± 0.6 | 68.5 ±1.5 | - 67.4 ± 4.9 | 88.6 ± 2.1 | (2.94 ± 0.07) ´10-16 |
| THF | 0.207 | 62.6 ± 0.7 | 21.4 ± 0.3 | 60.1 ±0.7 | - 77.4 ± 2.4 | 83.2 ± 1.1 | (2.65 ± 0.04) ´10-15 |
| Acetone | 0.355 | 56.7 ± 0.9 | 20.4 ± 0.4 | 54.2 ± 0.9 | - 82.4 ± 3.5 | 78.8 ±1.4 | (1.55 ± 0.03) ´10-14 |
| 3-Pentanol | 0.463 | 49.7 ± 1.2 | 19.3 ± 0.5 | 47.2 ± 1.2 | -89.2 ± 4.1 | 73.8 ± 1.7 | (1.17 ± 0.02) ´10-13 |
Table 2: Thermodynamic values for the isomerization of 4A4NAB obtained in each of the six solvents. The reported uncertainties are standard errors determined from replicate measurements.
The activation energies are then plotted against the relative polarity of the solvents (Figure 9). In all cases, the rate of isomerization increases as the solvent polarity increases with a concomitant decrease in the activation energy, enthalpy, entropy and free energy of activation for the cis-trans isomerization process (Tables 1 and 2) [20,23].
Overall, the data show that the rate of transition from cis to trans is strongly solvent dependent. Typically, solvent polarity has very little effect on the rate of isomerization when considering an inversion-based mechanism. A strong relationship between rate of reactions and solvent polarity points to an intermediate transition state that is considerably more polar than the cis conformation. This polar transition state is indicative of a rotational mechanism for the isomerization of 4A4NAB [21,22,24]. This result is expected considering the strong electron withdrawing group (the nitro group) on 4A4NAB and is corroborated by computational studies [22,25,26]. The isomerization mechanism of 4A4NAB can thus be defined as a rotation around the azo bond assisted by a linearization of the angle of the ring attached to the electronwithdrawing substituent. The extent of such assistance depends on the polarity of the solvent, being less important as the polarity increases [8]. Indeed, as the solvent polarity increases, a noticeable decrease in the activation energy, entropy, enthalpy and Gibbs free energy of activation for the cis-trans isomerization process is observed, a result typical of para-donor, para-acceptor type azobenzenes. However, for some azobenzenes, a deviation from linearity of the rate constants or activation energy versus solvent polarity, particularly at low polarity, may indicate a change in the isomerization mechanisms, from rotational for polar solvents to inversion mechanisms for non-polar solvents [20,27]. While structural differences and types of substituents play an important role in the isomerization mechanisms of azobenzenes, a detailed mechanistic study is required to delineate the different steps involved.
The authors wish to thank the Collegiate Science and Technology Entry Program (CSTEP) at SUNY Potsdam for funding this project and the Chemistry department for providing some of the materials used in the experiments.