Journal of Cell Signaling
Open Access
ISSN: 2576-1471
ISSN: 2576-1471
Research Article - (2019)Volume 4, Issue 4
Objective: Preeclampsia is an obstetric complication wherein trophoblast invasion is decreased and high levels of serum Gas6 protein are present. Our objective was to assess members of the mTOR family that are plausibly involved in the invasion of trophoblast cells resulting from Gas6/AXL activation.
Methods: Human first trimester cell line; (SW71), placental choriocarcinoma cell line (Jeg-3) and pulmonary alveolar type II-like cell line (A549) were treated with Gas6 or Gas6 and R428 (AXL receptor inhibitor) for 24 hours and real time cellular invasion was determined. Western blots were utilized to assess AXL receptor expression in treated and control cells. An Akt/mTOR phospho protein multiplex approach was used to determine the activity of mTOR related proteins.
Results: As compared to controls, cell treatments showed: 1) decreased SW71 invasion and increased Jeg-3 and A549 invasion with Gas6, 2) reversal of invasive properties when Gas6 and R428 were co-administered in A549 and SW71 cells, 3) increased expression of pAXL cells with Gas6, 4) decreased pAXL expression when Gas6 and R428 were added, 5) a trend of decreased phosphorylation of mTOR related proteins by Gas6 in trophoblasts, and 6) a trend of increased phosphorylation of mTOR related proteins by Gas6 in lung cancer cells.
Conclusions: These experiments revealed that mTOR family members are involved in the activation of AXL receptors during the regulation of cell invasion. The results provide important insight into the mechanism of AXL regulation of trophoblast cell invasion and identify potential avenues that may help in the understanding of obstetric complications associated with the aberrant invasion of trophoblast cells.
SW71: Swan 71 First Trimester Trophoblast Cells; Jeg-3: Placental Choriocarcinoma Cells; A549: Pulmonary Alveolar Type II-like Lung Adenocarcinoma Cell Line; Gas6: Growth Arrest Specific 6; AXL: AXL Tyrosine-Protein Kinase Receptor; mTOR: The Mammalian Target of Rapamycin; p70S6K: Ribosomal Protein S6 Kinase Beta-1; IRS1: Insulin Receptor Substrate 1; GSK3α: Glycogen Synthase Kinase-3 Alpha; GSK3β: Glycogen Synthase Kinase-3 Beta; Akt: Protein Kinase B; PTEN: Phosphatase and Tensin Homolog; IR: Insulin Receptor; IGF1R: Insulin-Like Growth Factor 1 Receptor; RPS6: Ribosomal Protein S6; TSC2: Tuberous Sclerosis Complex 2.
Trophoblasts are specialized epithelial cells that are critical for a successful pregnancy. During pregnancy, trophoblasts invade the placenta and replace the maternal endothelial cells that line the uterine spiral arteries, modifying them to become high capacity and low resistance vessels to meet increasing blood flow demands [1,2]. This remodeling allows for greater oxygen and nutrient exchange, and appropriate fetal development [1]. Placental and fetal development are impacted by aberrant trophoblast invasion associated with obstetric complications such as preeclampsia (PE). In PE pregnancies, shallow invasion of trophoblasts fails to convert uterine spiral arteries into high capacity and low resistance vessels, which leads to decreased blood flow [1-3]. This could result in local hypoxia which may affect placental and trophoblast development and function [1-4].
Growth arrest specific 6 (Gas6) is a vitamin K dependent protein originally discovered in embryonic mouse fibroblasts [5,6]. This protein is expressed in the lung, heart, kidney, intestine, and placenta [6]. Gas6 binds to the Tyro3, AXL, and Mer tyrosine kinase receptors but it has the highest affinity for AXL receptor [7] AXL receptors are broadly expressed in several tissues including muscle, liver, gallbladder, intestines, kidney, testes, endometrium, and placenta [8-14]. A diversity of signaling molecules associated with Gas6 and AXL control cell survival, inflammatory signaling, cell migration, and proliferation [7,15,16]. The Gas6/AXL pathway has been postulated in pathophysiological diseases including chronic immune disorders, cardiovascular diseases, and cancer [17,18]. AXL activation was shown to play a pivotal role in enhancing motility and invasiveness of cells [19,20]. Recent research has shown that there is an increase of Gas6 protein in the serum of preeclamptic women, but the role of Gas6 signaling in trophoblast invasion has yet to be fully determined [21,22].
The mammalian target of rapamycin (mTOR) protein is a phosphatidylinositol kinase-regulated protein kinase that regulates cell growth in response to nutritive insults and growth factors [23]. The mTOR pathway is important in regulating cell survival, metabolism, and growth [24-26]. As such, this pathway may be important in regulating and controlling cell invasion [1]. As supported by numerous studies, the mTOR pathway has also been linked to cell invasion in many different carcinomas and cancer metastases including a nonsmall cell lung cancer model [27]. More specifically, inhibition of mTOR activation has been associated with decreased trophoblast invasion [1], and mTOR activation has been associated with AXL activation in some diseases [28,29].
Although reports have shown aberrant trophoblast function during PE, there are no reports regarding trophoblast invasion and the role of Gas6/AXL signaling in the context of mTOR associated proteins in these cells. We hypothesize that Gas6/AXL signaling and the mTOR pathway play active roles in regulating cell invasion and that abnormal expression of these pathways could be associated with the development of obstetric complications such as PE.
To test our hypothesis, we treated trophoblast cells with Gas6 and R428 (a known inhibitor of AXL receptor), allowed them to invade through an invasion chamber, and analyzed protein expression by western blotting. For experimental comparison, we also used a lung cancer cell line (A549) to which AXL activation has been shown to play a pivotal role in enhancing motility and invasiveness [30,31]. Using this pulmonary cell line adds to the functional impact of AXL and the degree to which it causes invasion.
Cell culture
An immortalized first trimester trophoblast Swan71 cell line (SW71), placental choriocarcinoma cell line (Jeg-3), and a pulmonary alveolar type II-like lung adenocarcinoma cell line (A549) were used in this study. The SW71 cell line was a generous gift from Dr. Gil Mor at Yale University. Jeg-3 and A549 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA). All cell lines were maintained and cultured in RPMI medium (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. Cells were plated and treated in 6 well plates and used for protein analysis.
Cell treatments
Control cells were incubated in media alone (n=10); the first group of experimental cells were treated with media supplemented with 200 or 600 ng/mL Gas6 (n=10; R and D Systems, Minneapolis, MN), and a second group of experimental cells were treated with media supplemented with both 600 ng/ml Gas6 and 600 ng/ml R428 (n=10; AXL inhibitor; Selleckcheme, Houston, TX). Treatments were conducted for 24 hours. Cells were then detached using Trypsin EDTA (0.05%; Thermo Fisher, Waltham, Massachusetts) for invasion analysis or lysed used RIPA buffer (Thermo Fisher, Waltham, Massachusetts) for immunoblotting studies.
Real time cell invasion determination
Real time cell invasion was performed as previously described [32,33]. Briefly, additional cell cultures were conducted and invasions were assessed in 16 well-CIM-Plates (n=9; ACEA Biosciences, San Diego, CA). These plates are composed of an upper chamber coated with type IV collagen (1:40; Corning, Corning, NY) and a lower chamber with 10% FBS RPMI. Cells were plated in the top chamber at a concentration of 20,000 cells/well in 2% FBS RPMI in a total volume of 100 μL in the presence of Gas6, or Gas6 and R428. The cells were then placed in the RTCA DP instrument and invasion readings were taken every 15 minutes for 24 hours.
Immunoblotting
Western blot analysis was used to determine the expression levels of total AXL in control and treated cell groups as previously described [34]. Cell lysates (50 μg) were separated on a 10% SDS-Page gel and transferred onto nitrocellulose membranes. The membrane was blocked and incubated with antibodies against Gas6 (1:150; Abcam, Cambridge, MA), AXL (1:200; Cell Signaling Technology, Danvers, MA) or β actin (1:400; Santa Cruz Biotechnology, Dallas, Texas). Membranes were then incubated with secondary IRDye antibodies (1:10,000, 680RD donkey-anti goat and 680RD donkey-anti rabbit; LICOR Lincoln, NE) at room temperature for an hour. Membranes were finally developed on a Li-COR Odyssey CLx. All results were normalized to β actin readings. Fluorescence densities were determined and comparisons were made between treated and control groups.
Immunofluorescence (IF)
Immunofluorescence (IF) was performed in control and treated cells. In brief, cells were fixed in ice cold acetone for 20 minutes and washed with 1x PBS. Slides were then blocked with 4% horse serum for one hour at room temperature. Blocking was followed by overnight incubation at 4°C with a primary antibody for pAXL (Invitrogen, Carlsbad, CA) at a 1:100 concentration. Slides were then incubated for 1 hour with 1:500 donkey-anti mouse IRDye 800CW (LICOR; Lincoln, NE). 4’, 6-diamidino-2-phenylindole, dihydrochloride (DAPI) was used for counterstaining prior to mounting with glass coverslips. Slides were viewed using a FITC excitation and emission filter and imaged at 20X magnification. Quantification of fluorescence was performed using Image J software (n=9).
Akt/mTOR phosphoprotein magnetic bead cell signaling multiplex assay
Treated and control SW71 and A549 cell lysates were used in multiplex Akt/mTOR phosphoprotein 11-plex Magnetic Bead Kits (EMD Millipore, Billerica, MA) to detect changes in phosphorylated p70S6K (Thr412), IRS1 (Ser636), GSK3a (Ser21), GSK3b (Ser9), Akt (Ser473), PTEN (Ser380), IR (Tyr1162/Tyr1163), IGF1R (Tyr1135/ Tyr1136), RPS6 (Ser235/Ser236), TSC2 (Ser939), and mTOR (Ser2448). The Milliplex Multiplex Assay was performed as previously described [35]. Briefly, cells were separated into three groups; control, Gas6 and Gas6+R428. Beads were added to the cell lysates as well as detection antibodies. Cell lysates were incubated with antibodyconjugated magnetic beads overnight at 4°C. Bead-complexes were then read on a Magpix multiplex platform (Luminex Corporation, Austin, TX). Median fluorescent values were recorded from a minimum of 80 beads that were used for data analysis. Standard curves and data analysis was performed using Milliplex Analyst 5.1 software (Millipore Corporation, Billerica, MA).
Statistical analysis
End points obtained from control, Gas6 and Gas6+R428 treated cells were statistically compared. Differences in means ± SE were specifically assessed using the Mann-Whitney U-test. Significant differences between the groups were noted at p<0.05.
Gas6 and cell invasion
The AXL receptor has been associated with cell invasion [10,36,37]. Our first objective was to determine the effects of Gas6 on the invasion of SW71, Jeg-3 and A549 cells. Results from the real-time invasion assay showed that the addition of 200 or 600 ng/ml recombinant Gas6 induced a significant decrease (1.2-fold, 2.2-fold; p<0.002) in SW71 invasion during 24 hours of culture (Figure 1A). Interestingly, Jeg-3 cells showed a 1.3-fold decrease (p<0.0002) in cell invasion with 200 ng/ml of Gas6 while there was a 1.5-fold increase (p<0.0002) observed when treated with 600 ng/ml (Figure 1B). Invasion in A549 cells was increased with Gas6 at both 200 mg/ml and 600 ng/ml (1.7-fold, 3.6- fold; p<0.03; Figure 1C).
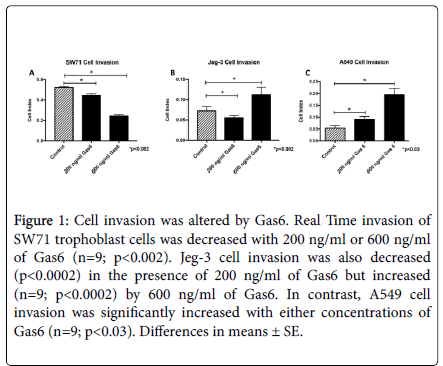
Figure 1. Cell invasion was altered by Gas6. Real Time invasion of SW71 trophoblast cells was decreased with 200 ng/ml or 600 ng/ml of Gas6 (n=9; p<0.002). Jeg-3 cell invasion was also decreased (p<0.0002) in the presence of 200 ng/ml of Gas6 but increased (n=9; p<0.0002) by 600 ng/ml of Gas6. In contrast, A549 cell invasion was significantly increased with either concentrations of Gas6 (n=9; p<0.03). Differences in means ± SE.
There was a higher fold change with 600 ng/ml for most of the cell types so we opted to continue to use 600 ng/ml as the concentration of Gas6 for the remainder of our studies. To determine if the AXL receptor is involved in invasion changes induced by Gas6, cells were treated with R428, an inhibitor of AXL receptor activation. In SW71 trophoblast cells, co-treatment with Gas6 and R428 increased cell invasion to basal levels (Figure 2A). In contrast, invasion of Jeg-3 trophoblast cells was not reduced when AXL was inhibited in the presence of Gas6 (Figure 2B). The increased invasion observed by Gas6 in A549 was completely rescinded when cells were treated with Gas6 and R428 (Figure 2C). These data suggest a key role of Gas6/AXL in the control of cell invasion in trophoblast and A549 cells.
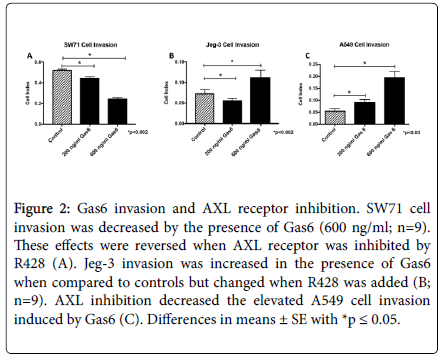
Figure 2. Gas6 invasion and AXL receptor inhibition. SW71 cell invasion was decreased by the presence of Gas6 (600 ng/ml; n=9).These effects were reversed when AXL receptor was inhibited by R428 (A). Jeg-3 invasion was increased in the presence of Gas6 when compared to controls but changed when R428 was added (B;n=9). AXL inhibition decreased the elevated A549 cell invasion induced by Gas6 (C). Differences in means ± SE with *p ≤ 0.05.
AXL activation and Gas6
Following cell invasion analysis, we next determined basal levels of Gas6 in the cell. Densitometric analysis of bands revealed Gas6 protein to be present in all the cells studies with highest expression see in the SW71 trophoblast cells (Figure 3A). We next sought to determine expression of the AXL receptor in cells treated with Gas6 and R428. AXL receptor was increased when Gas6 was added in both SW71 and A549 (2.0-fold, 2.7-fold; p<0.03; Figure 3B and 3D). Increased levels of AXL were reversed when these cell types were co-treated with both Gas6 and R428 (Figure 3B and 3D). In contrast, Jeg-3 trophoblast cells did not change AXL levels with either Gas6 alone or with both Gas6 and R428 (Figure 3C). To confirm AXL receptor activation and its inhibition by R428, immunofluorescence was performed for phosphorylated AXL (pAXL). We observed elevated staining intensity in all treated cell types with Gas6 and a significant reduction of pAXL with R428 treatment (Figure 4). When fluorescence was quantified, we discovered a significant difference (p<0.05) in all cells when comparing Gas6 and Gas6+R428 treatment (Figure 4). Untreated control cells showed negligible pAXL activation. The data suggest a role for Gas6 induced AXL activation in the control of cellular invasion.
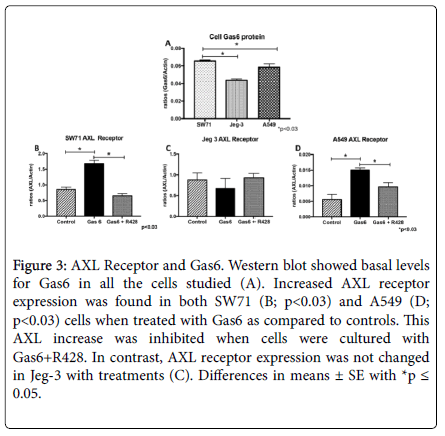
Figure 3. AXL Receptor and Gas6. Western blot showed basal levels for Gas6 in all the cells studied (A). Increased AXL receptor expression was found in both SW71 (B; p<0.03) and A549 (D; p<0.03) cells when treated with Gas6 as compared to controls. This AXL increase was inhibited when cells were cultured with Gas6+R428. In contrast, AXL receptor expression was not changed in Jeg-3 with treatments (C). Differences in means ± SE with *p ≤0.05.
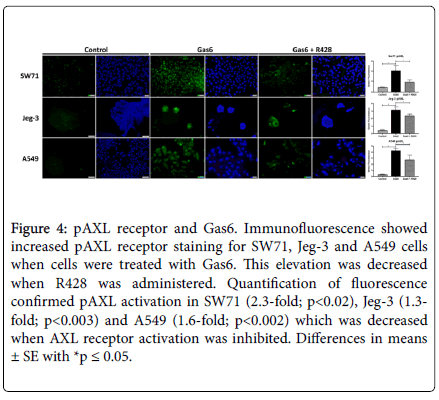
Figure 4. pAXL receptor and Gas6. Immunofluorescence showed increased pAXL receptor staining for SW71, Jeg-3 and A549 cells when cells were treated with Gas6. This elevation was decreased when R428 was administered. Quantification of fluorescence confirmed pAXL activation in SW71 (2.3-fold; p<0.02), Jeg-3 (1.3-fold; p<0.003) and A549 (1.6-fold; p<0.002) which was decreased when AXL receptor activation was inhibited. Differences in means ± SE with *p ≤ 0.05.
Phospho (P) mTOR related proteins and AXL
The mTOR pathway has been correlated with cell invasion [1], and reports have linked AXL activation with the mTOR pathway [28,29]. We wanted to determine the activity for some of the proteins associated with the mTOR pathway during treatment of cells with Gas6 or Gas6 and R4 [28]. An Akt/mTOR 11-plex magnetic kit was used for these experiments. There was a general trend of significantly decreased activation of mTOR related phosphoproteins when trophoblast cells were treated with Gas6. GSK3β phosphorylation was decreased (1.3- fold; p<0.02) when trophoblasts were treated with Gas6 (Figure 5A). This decrease was maintained (1.4-Fold; p<0.006) even when AXL was inhibited by R428 (Figure 5A). In contrast, IGF1R was increased (1.8- Fold; p<0.0002) by Gas6 and this Gas6-induced increase was reversed by AXL (Figure 5B). IRS1 phosphorylation was not affected by Gas6 (Figure 5C).
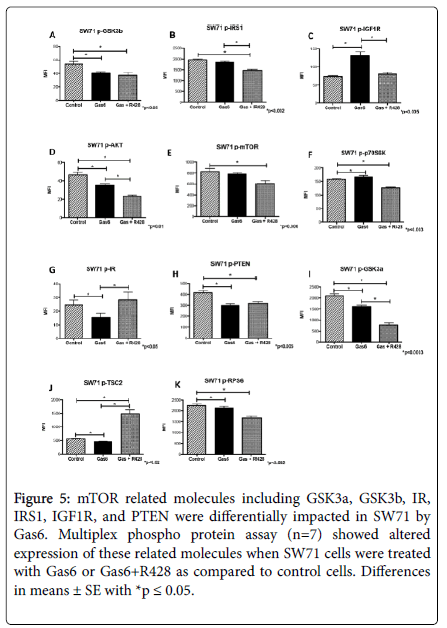
Figure 5. mTOR related molecules including GSK3a, GSK3b, IR, IRS1, IGF1R, and PTEN were differentially impacted in SW71 by Gas6. Multiplex phospho protein assay (n=7) showed altered expression of these related molecules when SW71 cells were treated with Gas6 or Gas6+R428 as compared to control cells. Differences in means ± SE with *p ≤ 0.05.
Akt phosphorylation was decreased (1.0-fold, p<0.01) by Gas6, while mTOR and p70SK6 was not affected when trophoblast cells were treated with Gas 6 alone (Figure 5D-5F). Interestingly, IR phosphorylation was decreased (1.6-Fold; p<0.05) by Gas6 and reversed when AXL receptors were inhibited (Figure 5G). PTEN and GSK3α phosphorylation was decreased (1.4-Fold, 1.3-Fold; p<0.004) by Gas6 in trophoblast cells (Figure 5H-5I); AXL inhibition was not sufficient to reverse the Gas6 effects on PTEN (1.3-Fold; p<0.0008) and even accentuated the decrease observed in GSK3α (2.7-Fold; p<0.0003; Figure 5H-5I). Similarly, TSC2 phosphorylation was decreased (1.2- Fold; p<0.02) by Gas6, but this decrease was reversed and even augmented (2.6-Fold; p<0.0002) when AXL was inhibited by R428 in these cells (Figure 5J). Interestingly, RPS6 phosphorylation was not affected by Gas6 in trophoblast cells (Figure 5K).
In the A549 cells, there was a general increase in the mTOR related phosphoproteins when cells treated with Gas6. p-GSK3β was significantly increased (1.5-fold; p<0.0002) in cells treated with Gas6, but this increase was slightly reduced (1.2-fold; p<0.005) when AXL was inhibited by R428 (Figure 6A). Similarly, Gas6 treatment significantly increased (1.1-fold; p<0.05) the expression of p-IGF1R, but this increase was reversed (1.2-Fold; p<0.002) when AXL was inhibited by R428 (Figure 6B). There was an increase in phosphorylation for both IRS1 (1.2-Fold; p<0.02) and Akt (1.3-Fold; p<0.007), however this increase was not reversed when AXL inhibitor was added to Gas6 treated cells (Figure 6C-6D). Interestingly mTOR phosphorylation was not affected in A549 cells treated with Gas6 (Figure 6E). In contrast, there was an increase in p70SK6 phosphorylation (1.2-Fold; p<0.009) by Gas6 that was reversed to basal levels when AXL receptor was inhibited by R428 (Figure 6F). Unexpectedly, we observed a 78-fold increase (p<0.0002) in IR phosphorylation when cells were treated with Gas6 (Figure 6G). This increase was reduced to a 29-fold (p<0.004) when R428 was added to the Gas6 treated cells (Figure 6G).
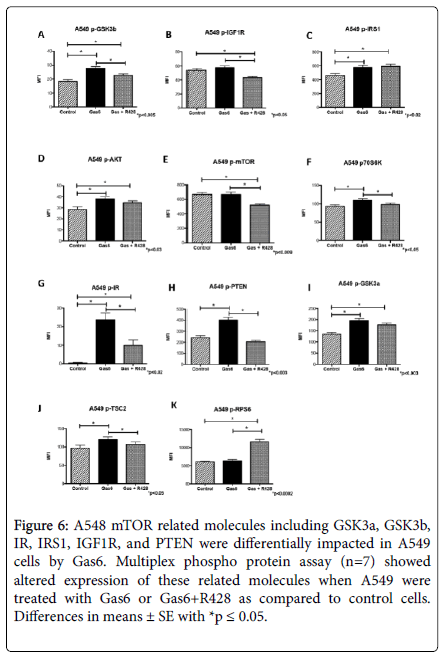
Figure 6. A548 mTOR related molecules including GSK3a, GSK3b,IR, IRS1, IGF1R, and PTEN were differentially impacted in A549 cells by Gas6. Multiplex phospho protein assay (n=7) showed altered expression of these related molecules when A549 were treated with Gas6 or Gas6+R428 as compared to control cells.Differences in means ± SE with *p ≤ 0.05.
GSK3α was increased by Gas6 (1.4-Fold; p<0.0003), but R428 had no effect in the increase observed by Gas6 in these cells (Figure 6H). Similarly, PTEN was increased by Gas6 (1.2-Fold; p<0.03) but returned to basal levels when AXL was inhibited (Figure 6I). There was an increase for TSC2 with Gas6 (1.2-Fold; p<0.03) that was completely reversed in the cells treated with Gas6 and R428 (Figure 6J). In contrast, RPS6 was not affected by Gas6 treatment (Figure 6K).
Proper placental development is critical for a successful pregnancy and a healthy placenta mediates important steps such as implantation, maternal blood flow through the placenta, and delivery of nutrients to the fetus [38]. Necessary for delivering nutrients and oxygen to the fetus are trophoblast cells that invade maternal tissue and remodel the restricted arteries to high volume, less resistant vessels. This process is compromised during complicated pregnancies such as preeclampsia and intrauterine growth restriction and each could affect fetal development [2,38]. Insights into the mechanistic pathways that contribute to these pathologies are incomplete. Studies examining the processes and mediators that influence trophoblast invasiveness may be instrumental in providing novel targets for future therapies that combat these obstetric complications.
A significant mediator of cell invasion is the tyrosine kinase receptor AXL which is expressed in the placental trophoblast [14]. Gas6 binding with AXL leads to AXL activation, which triggers a host of responses associated with cell survival, growth, aggregation, migration, invasion and inflammation [7,39-43]. In order to determine the effects of AXL receptor differences and its influence on invasion, trophoblast cells and lung cancer cells were assessed for invasiveness following exposure to Gas6. We observed that Gas6 was sufficient to reduce invasion of SW71 trophoblast cells. This reduction was reversed using a commercially available AXL inhibitor (R428). In contrast, Jeg-3 cells invasion was increased in the presence of Gas6 and this invasion was not affected by inhibition of AXL receptor. Lung cells treated with Gas6 also had increased cell invasion. This difference in invasion was rescinded when the AXL receptor was inhibited. The invasive difference between the trophoblast cells could be explained by the fact that Gas6 has the highest affinity for the AXL receptor but can also bind to the Tyro3 and Mer tyrosine kinase receptors [6,7]. These binding affinities plausibly suggest that perhaps cell invasion is not completely regulated by Gas6/AXL signaling in Jeg-3 cells. These results confirm the cell type dependent role of AXL activation in cell invasiveness. To elucidate the ability of the Gas6/AXL interactions to activate the mTOR pathway, we screened mTOR mediators in SW71 and A549 cell types. First, analysis of classical mTOR proteins revealed that mTOR phosphorylation was decreased by Gas6 and the decrease was not reversed by R428. Although these results were initially enigmatic in terms of Gas6 signaling, they suggest that alternative mechanisms independent of Gas6/AXL signaling may be involved in the phosphorylation of mTOR. Although unexpected, mTOR independent regulation during AXL activation was previously shown in other systems suggesting a different mechanism could be active in these cells [44].
Similar to observations for mTOR phosphorylation, p70SK6 activation via the mTOR pathway was decreased by Gas6 with no differences in activation following AXL inhibition in the trophoblast. In contrast, we observed an increase in its phosphorylation in lung cells that was reduced when AXL was inhibited, suggesting that p70SK6 could be one of the molecules involved in increased invasiveness by Gas6/AXL signaling in these cells. Collectively these results suggest that in trophoblast cells, Gas6 is not able to decrease activation of mTOR and p70SK6, and that although this could have an effect in the Gas6 induced decreased invasion originally observed in these cells, the outcome is not dependent on the AXL receptor. In contrast, A549 activation of p70SK6 could be involved in the increased invasion seen with increased Gas6. Together, these results support the idea of cell type specific Gas6 regulation of invasion. While biochemical links may exist in pathways that govern invasion of placental cells and neoplasms [45], it is reasonable that placentation and cancer development differ in outcome and thus respond in different ways to triggers of invasion such as AXL.
Ribosomal S6 phosphorylation (RSP6) has been correlated with the initiation of protein synthesis and is known to be phosphorylated by p70SK6 [46]. It was not surprising to observe a similar pattern in this protein as the observed for p70SK6. However, SK6 was also expected to follow the same pattern, but did not fully mirror results for p70SK6 phosphorylation. In the A549 cells we observed that although p70SK6 was activated by Gas6, this did not correlate with activation of SK6 which suggests that perhaps in these cells SK6 phosphorylation is not associated with increased invasion regulated by Gas6. More importantly, as previously seen in other systems, this suggests an independent regulation of SK6 by p70SK6 in these cells [47]. Consistent with the levels of mTOR and p70SK6 observed in trophoblast cells, there was decreased phosphorylation of Akt that was not affected by the inhibition of AXL. In the lung cells, Akt phosphorylation was increased by Gas6, which was not changed with the addition of R428. This suggests that Akt activation is possibly involved in the invasion of both cell lines and that a different mechanism is in place controlling its activation, despite AXL receptors being inhibited.
Glycogen synthase kinase 3 alpha and beta (GSK3α and GSK3β) are highly conserved kinases involved in diverse cellular processes such as cell proliferation, apoptosis, invasion and inflammation [48-51]. They are known to have specific targets in certain diseases [49,50], and previous studies suggest that GSK3β can promote the invasion of both trophoblast and lung cancer cells [51-53]. In our study, we found that both GSK3α and GSK3β phosphorylation were decreased by Gas6 in trophoblast cells, and this decrease was not recovered when AXL receptor was activated in the presence of Gas6. In contrast, GSK3α and GSK3β phosphorylation in A549 cells was increased in the presence of Gas6, and the phosphorylation was reduced when cells were treated with both Gas6 and R428. This further demonstrates Gas6/AXLdependent signaling during invasive behavior and confirms the invasive properties that have been previously shown by these molecules [51,54].
A protein complex consisting of the TSC1 (also known as hamartin) and TSC2 (also known as tuberin) has been the shown to inhibit the mTOR pathway [55]. Phosphorylation of TSC2 leads to the dissociation and inactivation of this complex leading to mTOR pathway inactivation [55]. Gas6 treatment reduced TSC2 phosphorylation in trophoblast cells, but increased phosphorylation of TSC2 in A549 cells. The effects induced by Gas6 in both cell types were reversed when AXL was inhibited by R428 in the presence of Gas6. These findings suggested a role for the inactivation of the TSC1-TSC2 complex in the invasive responses exerted by Gas6 in a Gas6/AXL signaling dependent manner.
The Insulin Growth Factor (IGF) system comprises a dynamic network of proteins in which activation leads to multiple signaling pathways including trophoblast migration as well as survival and progression of several cancers [56,57]. The insulin receptor (IR) and IGF-I receptor (IGF-IR) are tyrosine kinase receptors involved in the malignant invasive transformation of cells [56,58,59] and the insulin receptor substrate-1 (IRS-1) proteins are adaptor proteins downstream of activated cell surface receptors implicated in cell invasion [60]. In trophoblast cells, IR activation was decreased by Gas6 while IGF-1R phosphorylation was increased. These changes seem to be AXL activation dependent as they were reversed when the cells were treated with Gas6 and R428. IR results were expected as there is a correlation between IR and invasion in trophoblast cells and invasion was decreased in the presence of Gas6. We also expected IGF-1R to decrease; however, we observed the opposite in trophoblasts treated with Gas6. This may have occurred in response to the decreased invasion by Gas6. Once AXL was inhibited, cell invasion increased so the cells no longer required sustained IGF-1R phosphorylation as observed with R428 treatment in trophoblast cells. When screening these proteins during A549 invasion, we observed that the phosphorylation of both IR and IGF-1R was increased with the addition of Gas6, and IR phosphorylation was significantly higher than the rest of the proteins studied. Due to the previously established correlation of these molecules with cancer progression, our results suggest that Gas6 has a role in the regulation of A549 invasion. The phosphorylation of IR and IGF-1R proteins was decreased when Gas6 and R428 was added to the cells suggesting AXL induces the activation of the IGF system leading to increased invasion in these cells. Phosphorylation of IRS1 was not affected by Gas6 in trophoblast cells but was increased in A549 cells suggesting it may only be involved during lung cancer invasion.
The lipid phosphatase PTEN is a tumor suppressor that is underexpressed in many human cells [61]. PTEN inhibits the migration and invasion of several cell types [61,62] and regulates tumor behaviors through its lipid phosphatase activity [1,63]. PTEN activity can be regulated by a variety of mechanisms including phosphorylation [64] at the Ser380 residue which leads to loss of phosphatase activity and increased cell invasion. In our study, we observed that PTEN phosphorylation in trophoblast cells was decreased by Gas6, but not by AXL inhibition. PTEN was increased when Gas6 was added to A549 cells. This suggests that PTEN phosphatase activity was increased in trophoblast cells and decreased in A549 which further supports the association between PTEN phosphorylation and the changes in invasion we observed following Gas6 treatment in these cells. PTEN phosphorylation was decreased with the addition of R428 in lung cancer cells, which correlates with the decreased invasion when AXL receptor was inhibited.
As previously mentioned, trophoblast invasion is critical for proper placentation and fetal development during pregnancy. In this study, we showed the interaction between Gas6/AXL signaling and mTOR related proteins in the invasion of diverse cell types. Although some of the protein targets seem to not be regulated by AXL, we did see a trend for a decrease in phosphorylation of the mTOR pathway proteins when invasion was reduced (trophoblast) which opposed a trend for increased phosphorylation when Gas6 was increased. We demonstrate how a combination of proteins from this pathway could be associated with the control of cell invasion through Gas6/AXL signaling in a cell dependent manner. A key concept clarified by these studies is that a general process such as invasion is intricately controlled in cell types with diverse properties and commitment. These results contextualize cancer progression while particularly pointing to new avenues that may help alleviate decreased invasion during obstetric complications and provide a better prognosis for both the mother and infant during placental disruption.
The authors would like to acknowledge Dr. Robert Hyldhal for his assistance and help with the Multiplex analyses.
This study was supported in part by grant NIH-7R00HD055054-06 and by BYU Mentoring Environment Grants.
The authors report no conflict of interest.
Citation: Hirschi KM (2019) The mTOR Family of Proteins and the Regulation of Trophoblast Invasion by Gas6. J Cell Signal. 4:203. doi:10.35248/ 2576-1471.19.4.203
Received: 15-Apr-2019 Accepted: 22-Apr-2019 Published: 29-Apr-2019 , DOI: 10.35248/2576-1471.19.4.203
Copyright: © 2019 Hirschi KM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.