Journal of Hematology & Thromboembolic Diseases
Open Access
ISSN: 2329-8790
ISSN: 2329-8790
Short Communication - (2023)Volume 11, Issue 2
Well differentiated liposarcoma is a frequently discerned, low grade, mesenchymal tumefaction composed of mature adipocytes depicting focal cytological atypia intermingled with stromal cells. The locally aggressive neoplasm depicts significant histological variability with an adipocytic component admixed with varying quantities of cellular constituents and emergence of diverse morphological subtypes. Biological behavior of the neoplasm is contingent to tumour location wherein deep seated lesions may dedifferentiate and exemplify distant metastasis. Atypical lipomatous tumour is synonymous with and morphologically or genetically identical to well differentiated liposarcoma. Peripheral neoplasms appear devoid of possible distant metastasis, may be appropriately alleviated with comprehensive surgical resection and can be scripted as ‘a typical lipomatous tumour’. Deep seated neoplasms confined to retroperitoneum, mediastinum or spermatic cord demonstrates enhanced possibility of dedifferentiation, localized tumour reoccurrence or tumour associated mortality and can be designated as ‘well differentiated liposarcoma’.
Well differentiated liposarcoma is a frequently discerned, low grade, mesenchymal tumefaction composed of mature adipocytes depicting focal cytological atypia intermingled with stromal cells. The locally aggressive neoplasm depicts significant histological variability with an adipocytic component admixed with varying quantities of cellular constituents and emergence of diverse morphological subtypes. Biological behavior of the neoplasm is contingent to tumour location wherein deep seated lesions may dedifferentiate and exemplify distant metastasis. Atypical lipomatous tumour is synonymous with and morphologically or genetically identical to well differentiated liposarcoma. Peripheral neoplasms appear devoid of possible distant metastasis, may be appropriately alleviated with comprehensive surgical resection and can be scripted as ‘a typical lipomatous tumour’. Deep seated neoplasms confined to retroperitoneum, mediastinum or spermatic cord demonstrates enhanced possibility of dedifferentiation, localized tumour reoccurrence or tumour associated mortality and can be designated as ‘well differentiated liposarcoma’. Comprehensive surgical eradication with tumour-free surgical perimeter of aforesaid neoplasms may be challenging to obtain. Characteristically, well differentiated liposarcoma enunciates ring, giant marker or rod chromosomes engendered from chromosome 12q13-15. Consequently, localized amplification of several adjoining genes such as MDM2 may ensue. Genetic amplifications of chromosome 12q12-21 may be discerned. MDM2 and CDK4 can be enunciated with PCR or FISH and aid segregation from diverse sarcomas. Amplification of carboxypeptidase M is observed. Genomic rearrangements and amplification of chromosome 10p11-14 along with complex rearrangements of chromosome 8 and chromosome 12 may ensue. Well differentiated liposarcoma commonly appears in adults with peak age of disease emergence at 40 years to 60 years. Children are exceptionally incriminated and lesions may be associated with Li-Fraumeni syndrome. Well differentiated liposarcoma emerges as a deep seated lesion confined to lower extremities, retroperitoneum, trunk, head and neck or spermatic cord, in decreasing order of frequency. Tumefaction may occur within diverse cutaneous and subcutaneous regions. No site of tumour emergence is exempt. Characteristically, well differentiated liposarcoma is well circumscribed and multi-lobulated. Gross tumour infiltration is exceptional. Cut surface of lipoma-like lesions is marbled and yellowish. Neoplasms with minimal adipocytic differentiation exhibit firm or fibrotic, whitish areas. Enlarged tumours enunciate peripheral adipose tissue necrosis. Dedifferentiated or non-lipogenic component appear as firm nodules or diffuse admixture with foci of low grade tumefaction. Cytological evaluation demonstrates enlarged cells imbued with multilobulated nuclei with commingled mature adipocytes. Bizarre tumour cells may be discerned. Microscopic features are contingent to tumour subtype. Generally, tumefaction is minimally cellular and composed of mature adipose tissue depicting adipocytes of variable magnitude intermingled with fascicles of fibrotic stroma traversing tumour parenchyma. Intervening fibrous tissue stroma is comprised of spindle-shaped cells incorporated with enlarged, hyperchromatic nuclei. Adipocytes may depict significant atypia although mitotic figures are uncommon. A typical adipocytic cells may frequently be confined to fibrous tissue septa or demonstrate perivascular distribution. Exceptionally, tumefaction can display foci of heterologous differentiation [1,2]. Well differentiated liposarcoma is categorized into distinctive histological subtypes as:
• Lipoma like this is commonly discerned, simulates lipoma and is constituted of mature adipocytes subdivided by fibrous tissue septa. Diffusely disseminated atypical cells are exceptional. Lipoblasts can be frequently discerned sclerosing subtype is comprised of minimal adipose tissue component. Collagenous fibrous tissue is intermingled with scattered adipocytes and atypical, multinucleated stromal cells. Tumefaction follows lipoma like subtype in frequency and is predisposed to emerge within para-testicular region or retroperitoneum.
• Inflammatory subtype is an exceptional variant wherein tumour parenchyma is infiltrated with chronic inflammatory cells as B lymphocytes and T lymphocytes with configuration of occasional lymphoid follicle. Intervening cellular, fibrocollagenous stroma depicts disseminated reactive lymphoid follicles sparsely intermingled with multinucleated, atypical adipose tissue cells. Aforesaid chronic inflammatory infiltrate may obscure constituent mature adipocytes. Retroperitoneal tumours are commonly discerned wherein segregation from non-lipogenic tumours may be challenging.
• Mixed subtype is constituted of commingling of aforementioned subtypes of well differentiated liposarcoma.
•Exceptional variants emerge as low grade lipoleiomyosarcoma or liposarcoma with focal leiomyosarcomatous differentiation. Smooth muscle component is variable and appears admixed with enlarged vascular walls. Tumefaction may undergo dedifferentiation [3,4].
•Well differentiated liposarcoma with low grade osteosarcoma like areas enunciates tumour foci simulating parosteal osteosarcoma or low grade central osteosarcoma (Figures 1 and 2, Table 1).
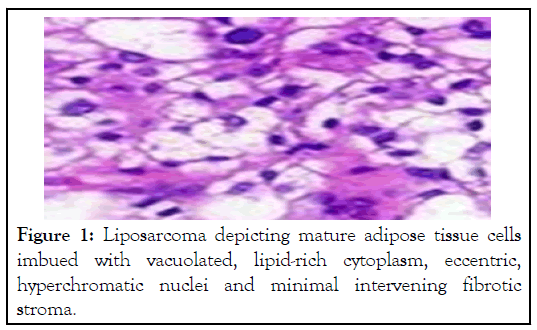
Figure 1: Liposarcoma depicting mature adipose tissue cells imbued with vacuolated, lipid-rich cytoplasm, eccentric, hyperchromatic nuclei and minimal intervening fibrotic stroma.

Figure 2: Liposarcoma delineating enlarged cells incorporated with multi-vacuolated, adipocerous cytoplasm, eccentric, hyperchromatic nuclei and mild inflammatory exudate.
| Tumour | Node | Metastasis |
|---|---|---|
| TX: Tumour cannot be assessed | NX: Lymph nodes cannot be assessed | |
| T0: No evidence of primary tumour | N0: Lymph node metastasis absent | M0: Distant metastasis absent |
| T1: Tumour ≤ 5 cm | N1: Regional lymph node metastasis present | M1: Distant metastasis present into lungs or diverse viscera |
| T2: Tumour between 5 cm to 10 cm | - | - |
| T3: Tumour between 10 cm to 15 cm | - | - |
| T4:Tumour >15 cm | - | - |
Table 1: TNM classification of soft tissue sarcoma-trunk, extremities, retroperitoneum.
Grading of soft tissue sarcoma is contingent to differentiation of neoplastic cells and mitotic activity scored from 1 to 3 and tumour necrosis scored from 0 to 2. Tumefaction is segregated into four grades denominated as:
• GX: Grade cannot be evaluated.
• G1: Total score of 2 or 3.
• G2: Total score of 4 or 5.
• G3: Total score of 6, 7 or 8. Soft tissue sarcomas are subdivided into diverse stages designated as:
• Stage I: Tumefaction is miniature and low grade (GX or G1).
• Stage II: Tumefaction is miniature and G2 or G3.
• Stage III: Tumefaction is enlarged and G2 or G3.
• Stage IV: Tumefaction disseminates to distant organs and viscera although initial tumefaction of variable magnitude or grade may display or appear devoid of regional lymph node metastasis.
Well differentiated liposarcoma is immune reactive to MDM2, CDK4, p16, S100 protein, CD34 and desmin. Well differentiated liposarcoma is immune non-reactive to HMB45. Well differentiated liposarcoma requires segregation from neoplasms such as castleman’s disease, hodgkin’s lymphoma, inflammatory myofibroblastic tumour, sclerosing mesenteritis or idiopathic retroperitoneal fibrosis, lipoblastoma, classic lipoma, lipomatous angiomyolipoma, massive localized lymphedema, myxoid liposarcoma, paraffinoma, spindle cell or pleomorphic lipoma, atypical spindle cell lipomatous tumour, dedifferentiated liposarcoma or lipomatous haemangiopericytoma. Retroperitoneal lipomas can be distinguished from well differentiated liposarcoma with cogent molecular investigations. Focal adipose tissue differentiation can be challenging to demarcate from native, intervening adipocytes wherein molecular techniques may be employed for distinction. Magnetic Resonance Imaging (MRI) of well differentiated retroperitoneal liposarcoma characteristically depicts mature adipose tissue with anomalous features as "stranding", indicative of thickened fascicles of fibrous tissue. Comprehensive surgical extermination with removal of broad perimeter of uninvolved tissue is optimal, recommended and appears to alleviate the neoplasm. Therapeutic outcomes are contingent to anatomic localization of tumours. Low grade neoplasms are accompanied by superior prognostic outcomes. Dedifferentiated neoplasms exhibit distant metastasis and decimated overall survival.
Comprehensive resection with eradication of pertinent surgical perimeter of retroperitoneal or centrically located neoplasms may be challenging. A foresaid tumefaction can frequently undergo tumours reoccurrence or dedifferentiation and engender tumour-associated mortality. Tumours cell aggregates confined to tumour periphery and sclerosing subtype is associated with decimated localized, recurrence- free survival. Subcutaneous or intramuscular tumefaction may undergo reoccurrence. Characteristically, dedifferentiation or distant tumour metastasis is absent. Possible occurrence of dedifferentiation is contingent to tumour location and duration of neoplastic representation.
Citation: Bajaj A (2023) The Oleaginous Transmogrification-Well Differentiated Liposarcoma. J Hematol Thrombo Dis. 11:530.
Received: 01-Feb-2023, Manuscript No. JHTD-22-18580; Editor assigned: 03-Feb-2023, Pre QC No. JHTD-22-18580(PQ); Reviewed: 17-Feb-2023, QC No. JHTD-22-18580; Revised: 24-Feb-2023, Manuscript No. JHTD-22-18580(R); Published: 03-Mar-2023 , DOI: 10.35248/2329-8790.22.11.530.
Copyright: © 2023 Bajaj A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited