
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2023)Volume 14, Issue 3
Background: With the development of antiretroviral treatment, the incidence of HIV-related illness and death has significantly decreased. Nevertheless, some individuals living with HIV who are taking Anti-Retroviral Therapy (ART) do not fully respond to the treatment in terms of immune restoration. This research aimed to examine the CD4+ T cell recovery pattern and factors influencing immune reconstitution among people with HIV on ART in Mekelle Hospital, Tigray, Northern Ethiopia.
Methods: A retrospective cross-sectional study involving HIV-positive patients receiving ART followup care was conducted in the hospital from January 2010 to July 2020 data was gathered through the utilization of a pre-tested structured questionnaire, which was administered by skilled data collectors. Statistical analysis was conducted using SPSS V. 20, with bivariate and multivariate analyses carried out to identify possible predictors of immune reconstitution after ART administration, 0.05% was considered as cut of level statistical significance.
Results: Out of the 424 study participants, 58% (248) were female, with a mean age of 37 ± 9. The ART follow-up lasted for a median duration of 60 months (IQR: 36-84), while the most recent median CD4+ T-cell count was 388 cells/μl (interquartile range: 254-527). The mean increase in CD4 cell count compared to the pre-ART level was 166 cells/μl of blood. Significant predictors associated with CD4+ T-cell recovery characterized by >350 cells/μl increments after long-term ART uptake were identified as follows: the age range of 25-34 years (Adjusted Odds Ratio [AOR] 2.62, 95% confidence interval [CI]: 0.82-8.35), baseline CD4+ T-cell count >200 cells/μl (AOR 3.53, 95% CI: 2.23-5.58), duration of ART follow-up of 12, 48, and 49 months or longer (AOR 8.053, 95% CI: 1.45-44.84; AOR 4.82, 95% CI: 1.16-20.11; AOR 6.36, 95% CI: 1.63-24.77), and TDF-3CT-Efv ART drug combination (AOR 2.29, 95% CI: 1.32-3.97).
Conclusion: The extent of immune recovery in people with HIV relies on the duration of their ART treatment and the CD4+ T-cell count at the time of initiation. Furthermore, individuals who received the TDF-3CT-Efv ART drug combination showed a faster restoration of CD4+ T-cells compared to those who were prescribed other ART drug regimens.
CD4+ T cell; HIV; Individuals; Antiretroviral treatment
Worldwide, HIV is one of the major threats to public health as it compromised the host’s immunity [1]. As of 2019, approximately 38 million individuals were living with HIV worldwide, with 25.4 million of them receiving Anti-Retroviral Therapy (ART) [2]. The global incidence and mortality rates for HIV in 2019 were 1.7 million and 690,000, respectively. The African region accounts for two-thirds of all HIV cases and in 2018, 470,000 people died from HIV/AIDS-related illnesses in Africa [3]. In Ethiopia, 718,498 individuals were reported to be living with HIV in 2017, with 426,472 of them receiving ART at a coverage rate of 59%. Despite the significant reduction in HIV-related morbidity and mortality with the introduction of combination ART therapy in 1996, some HIV-infected patients fail to achieve optimal immune response, resulting in AIDS-related deaths [4,5]. CD4+ T-cell count is a crucial immunological marker used to assess the immune function of HIV-positive individuals before treatment initiation and during ART follow-ups [6]. It is also the most important laboratory indicator of immune function, predicting disease progression and survival [7,8]. Although optimal viral RNA suppression through combination ART results in immune restoration for most patients, a significant number of ARTtreated patients do not achieve optimal immune recovery, leading to HIV/AIDS-related morbidity and mortality [9]. Additionally, some patients fail to experience immune reconstitution despite successful viral suppression. Nearly half of ART-treated patients fail to recover their CD4+ T-cell counts >500 cells/μl, and up to 16% to 20% may not reach CD4+ T-cell counts above 200 cells/ μl even with long-term therapy [10].
Numerous studies have produced controversial findings regarding immune restoration in HIV-infected individuals following a combination ART regimen [11-13]. According to some studies, the initial CD4 cell count influences how quickly the immune system recovers after receiving ART [8,14-16], while other studies have found little evidence to back up this assertion [17]. Furthermore, research indicates that younger people typically experience a faster rise in CD4 T-cell count after ART beginning [18,19]. However, there is currently no report on the pattern of CD4+ T-cell recovery and determinants of immune restoration following ART in the Tigray region of northern Ethiopia. To address this gap in knowledge, this study was conducted to evaluate the pattern of CD4+ T-cell recovery and determinants of immune restoration following ART at Mekelle Hospital in Tigray.
Study setting and design
From January 2010 to August 2020, a retrospective cross-sectional study was conducted among HIV-positive individuals on ART follow-up at Mekelle General Hospital, situated in the Tigray region of northern Ethiopia. This facility serves as the largest ART center in the region, catering to approximately 8399 HIVinfected individuals on ART follow-up. The study included all HIV-positive participants who were enrolled in ART follow-up, had a minimum of 6 months ART follow-up, and visited the ART clinic during the study period. The Tigray region comprises seven administrative zones, one special zone, 52 districts, and 799 Kebeles, with an estimated total population of 5.8 million people over an area of 50,078.64 square kilometers.
Inclusion criteria
The study included individuals who were HIV positive, aged 18 years or older, had a baseline CD4+ T-cell measurement, and had been on ART follow-up for at least six months.
Sample size and sampling technique
The sample size (n) for the study was determined using the single population proportion formula, assuming a CD4+ T-cell recovery rate of 50%. The resulting sample size was 385, but after factoring in a 10% non-response rate, the final sample size was calculated to be 424. To select study participants, a simple random sampling method was used. The sampling interval (K) was determined by dividing the expected number of HIV-positive individuals visiting the ART center during the study period, which was 1389. Using a lottery method, the first participant was selected among the first three patients with a sampling interval of every third individual added to the study list.
Data collection
Trained data collectors utilized structured questionnaires to gather information on various socio-demographic characteristics, such as sex, age, marital status, residence, education, occupation, and clinical data. In addition, patient record charts were examined to collect data on baseline CD4+ T-cell count, recent CD4+ T-cell count, combination ART regimens and length of time the patient had been following ART treatment [20].
Statistical analysis
SPSS V.20 was used to analysis data and for continuous variables, the median and interquartile values were used for summarization, while categorical variables were described using numbers and percentages. People with baseline CD4+ T-cell counts of 200 cells per liter or less or more were regarded to have significantly impaired immune systems [21]. According to their initial CD4+ T-cell counts, the majority of research patients exhibited advanced or severe immune suppression at the beginning of ART [22]. There was no clear consensus on the definition of optimal immune reconstitution, thus recent CD4+ T-cells were divided into two groups based on a cut-off value of <350 cells/μl and >350 cells/μl following the ART combination regimen. Bivariate and multivariate logistic regressions were used to identify factors associated with immune reconstitution. Bivariate analysis was used to determine the association between each predictor variable and the outcome variable, with all predictable variables having a p-value <0.05 being included in the multivariate model to identify predictable variables for CD4+ T-cell count >350 cells/μl. However, before building the final model, the multicollinearity effect was assessed using linear regression and a mean VIF >5 were used as the cut-off point. The final model was then evaluated for its goodness of fit using the Hosmer and Lemeshow test, with a p-value >0.05 indicating the best fit [23]. The association among the variables was determined by using odds ratio. Variables that showed statistically significant association at p<0.05 were considered independent predictors for CD4+ T-cell count >350 cells/μl.
Socio-demographic characteristics of the study participants
A total of 424 participants were eligible for this study, with 248 (58%) being female. The mean age of the participants was 37 ± 9 (SD) years, ranging from 19 to 68. The majority of participants were married (227, 54%) and lived in urban areas (406, 96%). Among the participants, 94 (22%) were housewives, while only 61 (14%) were civil servants (Table 1).
| Variables | N=424 | Frequency N (%) |
|---|---|---|
| Sex | Female | 248(58) |
| Male | 176(42) | |
| Age | 18-24 | 19(4.5) |
| 25-34 | 131(31) | |
| 35-44 | 180(42.5) | |
| ≥ 45 | 94(22) | |
| Residence | Rural | 18(4) |
| Urban | 406(96) | |
| Marital status | Single | 59(13) |
| Married | 227(54) | |
| Divorced | 91(22) | |
| Widowed | 47(11) | |
| Education | Illiterate | 106(25) |
| Elementary | 143(34) | |
| Secondary | 106(25) | |
| Certificate& above | 69(16) | |
| Occupation | Farmer | 30(7) |
| Merchant | 62(15) | |
| Civil servant | 61(14) | |
| Driver | 20(5) | |
| Housewife | 94(22) | |
| Others* | 157(37) |
Note: * indicates the other categories of the occupation.
Table 1: Socio-demographic characteristics of the study participants.
Clinical characteristics of the study participants
The majority, 414(98%), of the study participants were on the first-line ART combination regimen, and the remaining 10(2%) were on the second-line. Of the study participants, 233(55%) had a baseline CD4+ T-cell count >2000 cells/μl, ranging from 200-349 cells/μl, of blood. The majority of participants A had recent CD4+ T-cell count of 350-499 cells/μl of blood (Table 2). Approximately 57% of the individuals involved in the study had 49 months of ART follow-up, and 166 (40%) participants, were receiving a combination regimen of Tenofovir Disoproxil Fumarate (TDF), emtricitabine (3CT) and Efavirenz (Efv) as part of their ART treatment. One hundred sixty (38%) of the study participants had a baseline CD4+ T-cell count <200 cells/μl of blood.
| Characteristics- N=424 | CD4 cell count/ µl | Frequency N (%) |
|---|---|---|
| Baseline CD4 cell count | <100 cells/µl | 41(9.7) |
| 100-199 cells/µl | 123(29) | |
| 200-349 cells/µl | 233(55) | |
| 350-499 cells/µl | 22(5.1) | |
| ≥ 500 cells/µl | 5(1.2) | |
| Recent CD4 cell count | <100 cells/µl | 16(4) |
| 100-199 cells/µl | 55(13) | |
| 200-349 cells/µl | 105(24.8) | |
| 350-499 cells/µl | 131(30.9) | |
| ≥ 500 cells/µl | 117(27.6) | |
| CD4 cell count (median) | Baseline, median (IQR) | 222(160-310) |
| Recent , median (IQR) | 387.5(254-526.75) | |
| ART type | First line ART | 414(98) |
| Second line ART | 10(2) | |
| Duration of ART follow up (months) | ≤ 6 month | 13(3) |
| 7-12month | 20(5) | |
| 13-24 month | 45(11) | |
| 25-36 month | 40(9) | |
| 37-48 month | 66(16) | |
| ≥ 49month | 240(57) | |
| AZT-3CT-Nuv | 111(27) | |
| AZT-3CT-Efv | 48(12) | |
| 1st Line combination ART regimens | TDF-3CT-Nuv | 89(21) |
| TDF-3CT-Efv | 166(40) |
Table 2: Clinical characteristics of study participants.
Immune recovery after ART
The interquartile range (IQR) for the immunological failures was 53–88, with a median CD4+ T-cell count of 65 cells/μl of blood. The baseline CD4+ T-cell count for 70% (or 9%) of the research participants was less than 100 cells/μl. After six months of ART follow-up, 4% of these patients had CD4+ T-cell counts that were <100 cells/μl. Furthermore, less than 200 CD4+ T-cells per μl were present in 160 (38%) of the patients at baseline. Only 71 (18%) of them had a CD4+ T-cell count of fewer than 200 cells/ μl after 6 months of ART follow-up.
CD4+ T-cell recovery after the ART follow up
The median CD4+ T-cell count was 222.5 (IQR: 160-310) cells/ μl at the time of baseline. The median CD4+ T-cell count, however, was most recently measured at 388 (IQR: 254-527) cells/μl. The average number of months for ART follow-up was 60 (IQR: 36-84). The median CD4+ T-cell count rose from baseline to 6 months of ART follow-up by 69 cells/μl, reaching 319 (IQR: 113.5-698) cells/μl. Similar to this, after receiving ART for 12 months, the median CD4+ T-cell count rose by 153 cells/μl from the starting point to reach 403 (IQR: 289-470) cells/μl. Additionally, the median CD4+ T-cell counts increased throughout time, reaching 392, 417, 427, and 436 cells/μl after 24, 36, 48, and 49+ months of ART follow-up, respectively (Figure 1). Bivariate analysis revealed a statistically significant connection between baseline CD4+ T-cell count restoration to >350 cells/μl and the gender, age, employment, baseline CD4+ T-cell count, length of ART follow-up, and first-line ART regimen (P 0.05). To find potential factors linked to CD4+ T-cell recovery of >350 cells/μl, these variables were then included to a multivariate logistic regression model.
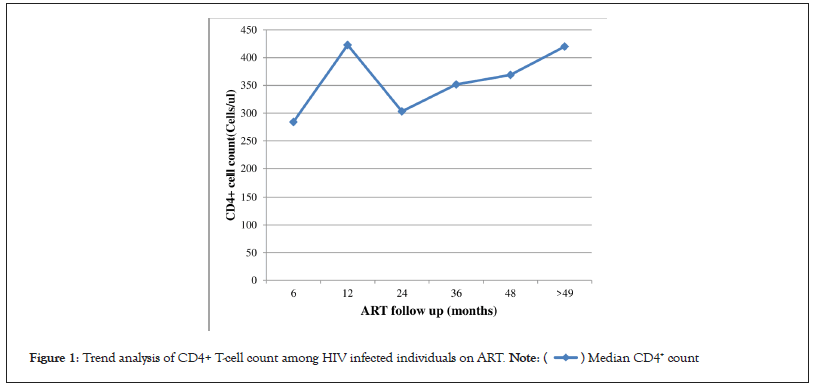
Figure 1: Trend analysis of CD4+ T-cell count among HIV infected individuals on ART. 
The multivariate analysis revealed that an age range of 25-34 years had an adjusted odds ratio (AOR) of 2.617 (95% CI: 0.821-8.348, P=0.001), a baseline CD4+ T-cell count >200 cells/μl had an AOR of 3.527 (95% CI: 2.231-5.576, P=0.001), and duration of ART follow-up at 12, 48 and 49+ months had AORs of 8.053 (95% CI: 1.446-44.842, P=0.017), 4.820 (95% CI: 1.155-20.111, P=0.030) and 6.362 (95% CI: 1.634-24.771, P=0.008), respectively. In addition, the ART regimen combination of TDF-3CT-Efv had an AOR of 2.288 (95% CI: 1.318-3.970, P=0.003). All these factors were found to be significantly associated with a CD4+ T-cell count increment of >350 cells/μl at P<0.05, as shown Table 3.
| Variables | CD4+ T-cell count >350 cells/µl | COR(95% C.I) | AOR(95% C.I) | P. value | |||
|---|---|---|---|---|---|---|---|
| Total (N= 424) | Yes N(%) | No N(%) | |||||
| Sex*+ | Female | 248(58) | 153(62) | 95(38) | 1.648(1.114-2.436) | 1.11(0.65-1.88) | 0.708 |
| Male | 176(42) | 87(49) | 89(51) | 1 | 1 | ||
| Age*+ | 18-24 | 19(4.5) | 13(68) | 6(32) | 2.683(0.939-7.661) | 2.62(0.82-8.35) | 0.104 |
| 25-34 | 131(31) | 92(70) | 39(30) | 2.921(1.680-5.076) | 3.01(1.58-5.71) | 0.001 | |
| 35-44 | 180(42.5) | 93(52) | 87(48) | 1.323(0.802-2.184) | 1.21(0.69-2.13) | 0.506 | |
| ≥ 45 | 94(22) | 42(45) | 52(55) | 1 | 1 | ||
| Residence | Rural | 18(4) | 8(44) | 10(56) | 1 | ||
| Urban | 406(96) | 232(57) | 174(43) | 1.667(0.644-4.311) | |||
| Marital status | Single | 59(13) | 33(56) | 26(44) | 0.788(0.361-1.720) | ||
| Married | 227(54) | 132(58) | 95(42) | 0.862(0.453-1.643) | |||
| Divorced | 91(22) | 46(50.5) | 45(49.5) | 0.634(0.310-1.300) | |||
| Widowed | 47(11) | 29(42) | 18(38) | 1 | |||
| Education | Illiterate | 106(25) | 59(56) | 47(44) | 1 | ||
| Elementary | 142(34) | 77(54) | 66(46) | 0.929(0.561-1.540) | |||
| Secondary | 106(25) | 60(57) | 46(43) | 1.039(0.604-1.788) | |||
| Certificate and above | 69(16) | 44(64) | 25(36) | 1.402(0.752-2.613) | |||
| Occupation*+ | Farmer | 30(7) | 11(37) | 19(63) | 0.478(0.213-1.070) | 0.40(0.16-1.02) | 0.06 |
| Merchant | 62(15) | 36(58) | 26(42) | 1.143(0.631-2.071) | 1.48(0.76 -2.90) | 0.249 | |
| Civil servant | 61(14) | 47(77) | 14(23) | 2.772(1.412-5.441) | 1.46(0.95-1.60) | 0.135 | |
| Driver | 20(5) | 7(35) | 13(65) | 0.445(0.168-1.174) | 0.28(0.09-1.86) | 0.127 | |
| House wife | 94(22) | 53(64) | 41(44) | 1.067(0.638-1.786) | 0.86(0.46-1.61) | 0.635 | |
| Others# | 157(37) | 86(55) | 71(45) | 1 | |||
| Baseline CD4count*+ | <200 cells/µl | 160(36) | 64(40) | 96(60) | 1 | 1 | |
| ≥ 200 cells/µl | 271(64) | 176(67) | 88(33) | 3.00(1.997-4.506) | 3.53(2.23-5.58) | 0.001 | |
| Type of ART | 1st line ART | 414(97.6) | 234(56) | 180(44) | 1.154(0.321-4.150) | ||
| 2nd line ART | 10(2.4) | 6(60) | 4(40) | 1 | |||
| Duration ART (months)*+ | ≤ 6 month | 13(3) | 5(39) | 8(61) | 1 | 1 | |
| 7-12month | 20(4.7) | 14(70) | 6(40) | 6.400(1.338-30.606) | 8.05(1.45-44.84) | 0.017 | |
| 13-24 month | 45(10.6) | 17(38) | 28(62) | 0.971(0.273-3.457) | 1.84(0.42-8.07) | 0.421 | |
| 25-36 month | 40(9) | 22(55) | 18(45) | 1.956(0.544-7.0208) | 2.52(0.58-10.92) | 0.218 | |
| 37-48 month | 66(15.6) | 36(55) | 30(45) | 1.920(0.568-6.490) | 4.82(1.16-20.11) | 0.03 | |
| ≥ 49month | 240(57) | 145(60) | 95(40) | 2.442(0.776-7.689) | 6.36(1.63-24.77) | 0.008 | |
| First line ART regimen*+ | AZT-3CT-Nuv | 113 (27) | 54(48) | 59(52) | 1 | 1 | |
| AZT-3CT-Efv | 48(11) | 28(58) | 20(42) | 1.530(0.773-3.026) | 1.55(0.73-3.31) | 0.259 | |
| TDF-3CT-Nuv | 89(21) | 47(53) | 42(47) | 1.223(0.701-2.133) | 1.37(0.72-2.58) | 0.335 | |
| TDF-3CT-Efv | 166(39) | 106(64) | 60(36) | 1.930(1.187-3.139) | 2.29(1.32-3.97) | 0.003 | |
Note: #Self-employee, daily work, student and commercial sex workers, *statistically significant at p<0.2, COR: Crude Odds Ratio,+variables included in multivariate model,astatically significant atP<0.05, AOR: Adjusted Odds Ratio.
Table 3: Bivariate and Multivariate analysis of variables associated with CD4+ T cell count >350 cells/µl among HIV infected individuals receiving ART.
HIV-positive people within the ages of 25-34 had a 2.6-fold higher chance than people outside of this age group of regaining their CD4+ T-cell count. Those with a baseline CD4+ T-cell count >200 cells/μl of blood were 3.5 times more likely to increase their count by 350 cells/μl than those with a count <200 cells/μl of blood. Additionally, individuals who underwent ART follow-up for 7-12 months, 37-48 months, and 49 months or longer were 8, 4.8, and 6 times more likely to recover their CD4+ T-cells count by 350 cells/μl of blood, respectively. Comparatively to people using other drug classes, those who’re taking the TDF-3CT-Efv ART combination regimen were 2.3 times more probably to increase their CD4+ T-cell count by 350 cells/μl of blood.
The median recent CD4+ T-cell count increased by 166 cells/ μl, or 388 cells/μl, among the research subjects. The median CD4+ T-cell count change found in the research was larger than that found in studies carried out in Nigeria and Ethiopia (Jigiga) [17,24], but less significant than that found in results from studies conducted elsewhere [14,19]. None of the patients attained >500 cells/μl, which is comparable with data from other trials, despite 56% of those with a baseline CD4+ T-cell count 200 cells/μl increasing it to >200 cells/μl after at least 6 months of starting ART [8,25-27]. However, a retrospective study found that 28.4% of individuals with a baseline CD4 count <200 cells/μl raised their count to 500 cells/μl or more in long-term ART follow-up [19], possibly due to variations in ART followup duration. Inadequate restoration of CD4+ T-cells may be associated with increased intrinsic CD4+ T-cell depletion and thymic and lymphoid differentiation dysfunction of HIV-infected individuals.
Following the start of ART, immunological failure was defined by a chronically low CD4+ T-cell count of less than 100 cells/μl [3,9]. The median CD4+ T-cell count for participants in this research who were classified as having immunological failure was 65 (IQR: 53-88) cells/μl. However, following the start of ART, 96% fewer HIV-positive people had a CD4 cell count of less than 100 cells/ μl in two or more consecutive assessments, which was comparable with previous Ethiopian research [19,28]. On the other hand, our result is higher compared to other studies [29,30], possibly due to variations in the immunological status of the study participants and ART follow-up duration. Moreover, low CD4+ T-cell count before ART initiation was the most determinant predictor of immunological failure among HIV patients [18], and persistent CD4+ T-cell cytolysis as a consequence of residual viral replication could be a reason for poor immune recovery during ART [31].
The median CD4+ T-cell count after 6 months of ART followup was 319 cells/μl, an increase of 69 cells/μl from the baseline, which was consistent with prior studies [23]. The median CD4+ T-cell count continuously grew to 403 cells/μl after 12 months of ART follow-up, an increase of 153 cells/μl over the initial level. Additionally, the median CD4+ T-cell count was 427 and 436 cells/μl at 48, 49 and beyond ART follow-up, respectively, demonstrating a continuing rise in the CD4+ T-cell count after ART beginning, in line with other studies of a similar kind [10,15,25].
In comparison to other age groups, individuals in the 25-34 years age group were more likely to recover their CD4+ T-cells after ART combination therapy with a significance of P=0.001. This suggests that younger HIV-infected individuals may have a higher probability of experiencing rapid CD4+ T-cell recovery following ART initiation compared to older age groups. This finding is consistent with previous studies conducted in Zimbabwe, Uganda, Ethiopia, France, and Australia [14,15,19,32,33]. In addition, additional studies have revealed that younger age groups with a baseline CD4+ T-cell count of less than 500 cells/ μl have showed quicker recovery and longer life expectancy in comparison to those with more than 500 cells/μl [5,18]. Studies done in China have also shown that people who begin ART at a younger age are more likely than older age groups to achieve optimum immunological recovery [16,26]. Additionally, several comparable studies have demonstrated that the age-related decline in CD4+ T-cell count following ART beginning is slowed [31,34-36]. However, according to certain research, younger HIVinfected people are less likely than older age groups (≥50 years) to achieve immunological reconstitution after receiving ART [37,38].
Individuals infected with HIV who had a baseline CD4+ T-cell count >200 cells/μl were more likely to increase their CD4+ T-cells above 350 cells/μl after ART initiation, with a P-value of 0.001. Long-term prospective cohort studies have suggested that ARTtreated individuals with a CD4+ cell count above 200 cells/μl at ART initiation tend to recover their CD4+ T-cells faster than those with a normalized CD4+ T-cell count [8,25,39-41]. A systematic review research also discovered that those with a baseline CD4+ T-cell count more than 350 cells/μl had a higher probability of immunological recovery following ART therapy, decreasing their risk of AIDS progression or passing away [42]. According to additional research, those who have greater pre-ART CD4+ T-cell counts are more likely to have optimal immune reconstitution when ART is started [15,16,26,33,43-45]. Evidence also indicates that early initiation of ART provides the best opportunity for CD4+ T-cell recovery and immune system preservation, leading to reduced CD4+ T-cell depletion, lower rates of AIDS events or opportunistic infections, and greater capacity for immune system reconstitution in general [22].
However, some studies have suggested that HIV patients with low pre-ART CD4+ T-cell counts experience rapid CD4+ T-cell recovery and have higher long-term mean CD4+ T-cell counts post-ART [11,14,30,35-50]. Additionally, a small number of studies have shown that the baseline CD4+ T-cell count does not significantly correlate with immunological restoration after ART therapy [27,50]. These discrepancies may be explained by differences in the timing of ART introduction (early vs. late), lower rates of AIDS-related complications, and greater rates of immune reconstitution system among individuals with low CD4 T-cell counts at ART commencement. The majority of research has found that a higher CD4+ T-cell count at baseline resulted in a stronger impact of ART on CD4+ T-cell recovery, albeit further well-designed prospective studies are required to corroborate these findings [13,19,24,30,46,47].
After starting ART for 12 months vs. the first 6 months, the CD4+ T-cell count grows greater (P=0.017). Studies conducted in the past reveal that CD4+ T-cell recovery was quick during the first year after starting ART [15,29,37]. CD4+ T-cell recovery continues to increase steadily after 12 months and reaches a plateau for optimal immune restoration [21]. CD4+ T-cell recovery is also optimal after 48 months and beyond of ART initiation. Prospective cohort studies in London and South Africa confirm increased CD4+ T-cells during ART follow-up [10,25]. Observational studies in China and Ethiopia also show improved immune recovery with longer ART duration [10,16,22,25,41,43,48]. The length of ART follow-up time is associated with continual CD4+ T-cell increase until reaching a plateau or threshold [38,44,45,47,48]. Patients on TDF-3CT-Efv 1st line ART regimen have higher CD4+ T-cell restoration compared to other regimens (P=0.003), consistent with reports from Australia and Ethiopia [15,43]. Tenofovir-based regimens lead to better CD4+ T-cell restoration compared to Zidovudine-based regimens [3,43,37,47]. TDF-based regimens are preferred for viral suppression and continual CD4+ T-cell improvement [49,50]. However, some studies contradict this finding, suggesting the need for further investigation [48]. Variables like sex, residence, marital status, occupation, and educational status do not significantly affect CD4+ T-cell recovery. The study lacks information on the clinical stage and virological status of patients at baseline and after ART administration.
To obtain maximal CD4+ T-cell recovery after commencing ART, Anti-Retroviral Treatment (ART) must be started at a CD4+ T-cell level >350 cells/μl. People who started ART earlier in life recovered more quickly and had reduced CD4+ T-cell depletion overall. Within the first year of ART commencement, the CD4+ T-cell count rises quickly and keeps increasing over the course of ART follow-up. Furthermore, individuals receiving a combination regimen of TDF-based ART demonstrated faster CD4+ T-cell recovery compared to those using other first-line categories. Therefore, the program should prioritize the TDF- 3CT-Efv regimen as the first-line ART combination for better outcomes. Additionally, ART initiation should be considered when CD4+ T-cell counts are above 350 cells/μl to optimize immune system restoration.
Ethical approval and consent to participate
The study obtained ethical clearance from the Tigray Health Research Institution’s Institutional Review Board (THRI-IRB). Additionally, an official written letter was obtained from the Tigray Health Research Institute. The research conducted in this study did not involve any direct contact with patients or patient samples. Instead, data were collected through a review of documents available in the THRI database. As a result, the requirement for obtaining informed consent from each patient’s medical records was waived.
The Tigray Health Research Institution Institutional Review Board (THRI-IRB) granted ethical clearance for this study, and an official letter was obtained from the Tigray Health Research Institute. Since the study did not involve direct contact with patients or their samples, data were collected by reviewing available documents in the THRI database. As a result, there was no need to obtain informed consent from individual patients’ medical records.
Consent for publication
All authors are agreed on the overall preparation of the manuscript and submission for publication on ADIS research and Therapy.
The data and materials are available with the authors and delivered upon a request.
Competing interests
All authors declared no conflict of interest.
Funding
As it is retrospective data, there was no specific fund for this study.
Acknowledgements
We would like to express our thanks and appreciation to the data collectors for their full responsibility and commitment. We would like also to acknowledge Mekelle Hospital Chief Executive Officer and ART clinic data manager for their permission and cooperation with the data collector team.
Author contributions
Conceptualization: Letebrhan Weldemhret, Abraham Aregay, Hadish Bekurtsion, Gebremicheal Gebreegziabher, Tsehaye Asmelash, Dawit Gebreegziabher Hagos.
Data curation: Letebrhan Weldemhret, Abraham Aregay, Hadish Bekurtsion, Gebremicheal Gebreegziabher, Tsehaye Asmelash, Dawit Gebreegziabher Hagos.
Formal analysis: Letebrhan Weldemhret, Abraham Aregay, Hadish Bekurtsion, Gebremicheal Gebreegziabher, Tsehaye Asmelash, Dawit Gebreegziabher Hagos.
Methodology: Letebrhan Weldemhret, Abraham Aregay, Hadish Bekurtsion, Gebremicheal Gebreegziabher, Tsehaye Asmelash, Dawit Gebreegziabher Hagos.
Writing–original draft: Letebrhan Weldemhret, Abraham Aregay, Hadish Bekurtsion, Gebremicheal Gebreegziabher, Tsehaye Asmelash, Dawit Gebreegziabher Hagos.
Writing-review and editing: Letebrhan Weldemhret, and Dawit Gebreegziabher Hagos.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Weldemhret L, Aregay A, Bekurtsion H, Gebreegziabher G, Asmelash T, Hagos DG (2023) The Pattern of CD4+ T Cell Recovery and Determinants of HIV Infected Individuals Receiving Highly Active Antiretroviral Treatment in Mekelle Hospital, Tigray Northern Ethiopia: A Retrospective Study. J Clin Cell Immunol.14:690.
Received: 16-May-2023, Manuscript No. JCCI-23-24307; Editor assigned: 19-May-2023, Pre QC No. JCCI-23-24307 (PQ); Reviewed: 05-Jun-2023, QC No. JCCI-23-24307; Revised: 12-Jun-2023, Manuscript No. JCCI-23-24307 (R); Published: 20-Jun-2023 , DOI: 10.35248/2155-9899.23.14.690
Copyright: © 2023 Weldemhret L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.