Advanced Techniques in Biology & Medicine
Open Access
ISSN: 2379-1764
ISSN: 2379-1764
Research Article - (2017) Volume 5, Issue 2
The aim of this work is to demonstrate the significance of the phenomena of light re-radiation and the electron excitation energy transfer from the donor to the acceptor in the hydrolysis reactions of glycoside and phosphodiester bonds in DNA, which is important for the functionality of cells in the norm and pathology and for the analysis of the quality of the double helix DNA for diagnostic purposes. It is shown that photons of the near-IR region of the spectrum excite the overtones of the large-amplitude valence vibration of water molecules in the 700-1500 nm spectral range. This causes the activation of electrolytic dissociation of water molecules with the formation of H+ and OH-, which is necessary for the hydrolysis reaction of chemical bonds in biological molecules. The application of the original nanoscale method of a laser induced fluorescence resonance energy transfer to a donor-acceptor intercalator pair for the quantitative and qualitative study of stability quality DNA double helix in a solution, in real time is shown in the following biologically important processes: photoirradiation, photodynamic effect and electron excitation energy transfer in strongly scattering environment (colloidal) with multiple scattering of light, i.e., in processes that can be successfully used in light therapy of cancer, dermatology, wound healing, etc.
Keywords: DNA, Intercalator, Resonance energy transfer, Reradiation
For the last several decades, there has been great interest in a bloodless surgery, especially for the treatment of malignant tumors and other growths has been observed. Such therapeutic treatments include: hadron, isotope, γ-radiation, microwave, chemo, fermento, photochemo, photothermo, plasma, etc.
A significant interest has appeared recently in the use of nonequilibrium atmospheric pressure cold plasma in medicine, especially in the treatment of cancer, the so-called "blood dialysis", healing wounds, and sterilization of expensive medical instruments, in which sterilization can’t be used at temperature higher than 40-50°C [1-9]. Besides, there are few papers in which non-equilibrium low-pressure plasma is used, mainly as a source of light in atomic emission analysis [10-16], in spite of the unique properties of inductively coupled low pressure plasma (1- 20 Torr).
We will shortly discuss properties of non-equilibrium reduced pressure plasma for the purpose of using it as a light source for light therapy in medicine.
If electron distribution function in plasma is close to Maxwell's distribution, then, in case of weakly ionized plasma, we can consider separately electronic Te and atomic Ta temperatures [17]. This occurs in case when the frequency of collisions of electrons with each other νee is much more then frequency of energy transfer from electrons to atoms νea, i.e.
νee >> νea
Thus
Ta << Te
Consequently, in both no equilibrium atmospheric pressure plasma and low pressure inductively coupled plasma atomization, gas molecule excitation happen almost exclusively through the collision of electrons, i.e., non-equilibrium plasma is characterized by relatively low electron energy ~1-1.5 eV [10]. Thus, nonequilibrium inductively coupled (110 MHz) low-pressure (Argon of 1-20 Torr) plasma is great source of line spectrum in near-infrared region of the spectrum (700-1500 nm) (Figure 1), which can be used in light therapy of malignant growths, both independently and with other methods of alternative therapy of cancer.
In fact, the photons of the near-IR region of the spectrum, exciting the overtones of the large-amplitude (733-01480 nm) valence vibration of water molecules, are capable of activating electrolytic dissociation of water molecules with the formation of H+ and OH-; this is necessary for the hydrolysis reaction of chemical bonds in biological molecules.
In fact, since cancer cells, unlike normal cells, are less structured, they are more accessible to a variety of chemicals. Chromatin and DNA of a cancer cell have a greater ability to bind water molecules as well as transition metal ions [19-21]. In addition, chromatin and DNA strongly scatter light, which leads to the Tyndall effect (multiple light scattering), which causes the dissipation of energy due to Raman scattering (inelastic). The latter is a catalyst for the electrolytic dissociation of a water molecule bound with chromatin and DNA, which leads, on the one hand, to the hydrolysis of phosphodiester and glycosidic bonds of DNA and proteolysis of proteins, on the other hand, to the start of programmed death of cancer cells (apoptosis), which is known to start when the protein material of the genome is damaged (Figure 2).
By selecting a mixture of gases (hydrogen, helium, nitrogen, oxygen, neon, argon and others), it is possible to obtain a spectrum of atoms in the range of 600-1500 nm, which will let radiation penetrate into the desired depth of the living tissue and induce hydrolysis reaction.
The aim of the work is to demonstrate the significance of the phenomena of light re-radiation and the electron excitation energy transfer from the donor to the acceptor in the hydrolysis reactions of glycoside and phosphodiester bonds in DNA, so important for the functioning of cells in the norm and in the pathology and also the analysis of the quality of the double helix DNA for diagnostic purposes.
Materials
DNA: In our tests we used the Calf thymus DNA (40% GC), ‘Sigma’. The concentration of nucleic acids was determined by UV absorption (spectrophotometer Specord M 40, Carl Zeiss) using molar extinction coefficients (ɛ=6600 cm-1 M-1 at λ=260 nm). The double helix structure of the polymers was proved by their hyperchromicity (>30%) and their typical thermal denaturation transition (measured in 0.01 M NaNO3, pH≅6.0). pH was checked by pH meter HANNA Instruments pH 213.
Intercalators: Acridine orange (AO) was purchased from ‘Sigma’. The concentration of the dye was determined colorimetrically using the molar extinction coefficients ε=68500 cm-1 M-1 at λ=492 nm. Ethidium bromide (EB) was also purchased from ‘Sigma’ and the concentration of the dye was determined colorimetrically using the molar extinction coefficients ε=5600 cm-1M-1 at λ=480 nm.
Instrumentation
Absorption spectra of DNA complexes with intercalators AO and EB were registered in real time (8 ms) using CCD spectrometer AvaSpec ULS 2048-USB2. It should be underlined that registration of fluorescence spectra excited by laser irradiation is necessary to carry out in real time as at such excitation of intercalators, AO in particular; its fast photo-oxidation takes place.
Diode laser SDL-475-100T (Shanghai Dream Lasers Technology Co., Ltd.) was used for irradiation and excitation (λ=457 nm with optical beam cross-section 2 mm and P=200 mW) of laser induced fluorescence spectra.
Photoirradiation was carried out in reactor with the fixed light beam in 1 cm rectangular fluorescent quartz cell. In the same cell with the interval of 5 min absorption spectra of irradiated solutions were registered by AvaSpec spectrometer. Before each absorption registration the cell was shut to protect the solution from photo irradiation.
Argon lamp: High-frequency, electrode less, low-capacity spectral lamp was used for irradiation of DNA. Very high frequency (VHF) generator (110 MHz) was used for the ignition of the argon lamp (Figure 3).
Fluorescence resonance energy transfer (FRET): The proposed laser-induced FRET method allows one to estimate the concentration of double helix areas with high quality stability applicable for intercalation in DNA after it was subjected to stress effect. It gives the opportunity to compare DNAs of 1) different origin; 2) with various damage degrees; 3) being in various functional states [22].
Figure 4 shows fluorescence spectra of binary and ternary AODNA and AO-EB-DNA complexes where the concentration of DNA changes the distance between donor AO and acceptor EB. AO and EB concentrations were constant and equal to 0.14 × 10-4 mol/L. DNA concentration varied from 0.5 × 10-4 to 5 × 10-4 mol/L per base pair (bp).
Figure 4: Fluorescence spectra of double and ternary DNA-AO and DNA-AOEB complexes where concentration of DNA changes the distance between donor AO and acceptor EB. [AO]-0.14·10-4 mol/L, [EB]-0.14·10-4 mol/L, DNA concentrations: (C0)-2.8·10-4 mol/L (P), (C1)-10∙10-4 mol/L (P), (C2)-8·10-4 mol/L (P); (C3)-6·10-4 mol/L (P); (C4)-4·10-4 mol/L (P); (C5)-2.8·10-4 mol/L (P);(C6)-1.4·10-4 mol/L (P); (C7)-10-4 mol/L (P).
Table 1 gives values for the efficiency EET=(1-qD/q0D) of AO intercalated in DNA depending on the distance between AO and EB given in bp units. The same table allows one to estimate the distance between donor and acceptor in correspondence with the values of EET evaluated from fluorescence spectra of FRET.
Figure 5 presents absorption spectra of AO and EB in free and intercalated in DNA states. The absorption spectra of the dyes, under their interaction with DNA duplex, move into the red region of spectrum and highly change their intensity. From photochemical point of view, we are highly interested in the decrease of absorption intensity (hypochromism) of AO and EB at their intercalation into DNA-highly ordered structure duplex. Hypochromism phenomena are connected with it light re-radiation and, in our case re-radiation to DNA, leads to strong increase by 4-5 orders scattering cross-section. Dye-intercalator in this case acts as a trap for the falling light beam, and DNA is an environment that can strongly scatter a significant part (10-20%) of the light, including inelastic scattering (Raman scattering) (Scheme 2).
Figure 6 shows the change of energy transfer efficiency from 13 bp to 7 bp from AO to ethidium bromide intercalated in DNA after 20 min of laser irradiation of the binary DNA-AO complex (Table 2).
Figure 7 shows the change of energy transfer efficiency from AO to EB intercalated in DNA after 120 min of argon lamp irradiation of the DNA solution. The ignition of the lamp occurs by VHF generator; the cuvette with the DNA solution is located in the focus of double condenser lens (Table 2). Preliminary results are encouraging and the study will be continued.
| EET | R (nm) | R (bp) | EET | R (nm) | R (bp) |
|---|---|---|---|---|---|
| 0.999 | 0.34 | 0 | 0.184 | 5.44 | 15 |
| 0.999 | 0.68 | 1 | 0.151 | 5.78 | 16 |
| 0.995 | 1.02 | 2 | 0.124 | 6.12 | 17 |
| 0.983 | 1.36 | 3 | 0.102 | 6.46 | 18 |
| 0.959 | 1.7 | 4 | 0.085 | 6.8 | 19 |
| 0.919 | 2.04 | 5 | 0.071 | 7.14 | 20 |
| 0.860 | 2.38 | 6 | 0.059 | 7.48 | 21 |
| 0.783 | 2.72 | 7 | 0.050 | 7.82 | 22 |
| 0.693 | 3.06 | 8 | 0.043 | 8.16 | 23 |
| 0.597 | 3.4 | 9 | 0.037 | 8.5 | 24 |
| 0.503 | 3.74 | 10 | 0.031 | 8.84 | 25 |
| 0.500 | 3.75 | 0.027 | 9.18 | 26 | |
| 0.416 | 4.08 | 11 | 0.024 | 9.52 | 27 |
| 0.341 | 4.42 | 12 | 0.020 | 9.86 | 28 |
| 0.278 | 4.76 | 13 | 0.018 | 10.2 | 29 |
| 0.226 | 5.1 | 14 | 0.016 | 10.54 | 30 |
Table 1: Efficiency of energy transfer EET=(1-qD/q0D) from AO donor to EB acceptor depending on the distance R between them
Figure 6: Influence of laser irradiation (λ=457 nm) on electron excitation energy transfer effectiveness from AO to EB intercalated in DNA. (A) AO-DNA; (B) AO-DNA-EB; (C) AO-DNA irradiation 20 min; D) AO-DNA irradiation 20 min + EB. [DNA]-7 × 10-4 mol/L (P), [AO]-0.14 × 10-4 mol/L, [EB]-0.14 × 10-4 mol/L, [NaNO3]-10-2 mol/L.
| Stress factor for DNA°C | EET (%) | RAO-EB(nm) | d | RstAO-EB/R0AO-EB(%) |
|---|---|---|---|---|
| Heating | ||||
| 20°C | 26 | 4.87 | 100 | |
| 50°C | 48 | 3.83 | 79 | |
| 60°C | 52 | 3.68 | 75 | |
| 70°C | 76 | 2.81 | 58 | |
| 80°C | 81 | 2.61 | 54 | |
| 90°C | 82 | 2.57 | 53 | |
| Boiling | ||||
| 20°C | 26 | 4.87 | 100 | |
| 100°C, 5 min | 80 | 2.65 | 54 | |
| 100°C, 10 min | 87 | 2.33 | 48 | |
| 100°C, 20 min | 95 | 1.80 | 37 | |
| Laser Irradiation | ||||
| DNA-AO | 26 | 4.87 | 100 | |
| DNA-AO (20 min) | 85 | 2.43 | 50 | |
| Ar lamp Irradiation | ||||
| DNA-AO | 10 | 6.50 | 100 | |
| DNA (120 min)-AO | 11.5 | 6.25 | 96 |
Table 2: Laser irradiation (λ=457 nm) and heating effects on EETa and RAOst-EB/RAO0-EBb.
Figure 7: Influence of Ar lamp irradiation on electron excitation energy transfer effectiveness from AO to EB intercalated in DNA. (A) DNA-AO; (B) DNA-irradiation 120 min - AO; (C) DNA-AO-EB; (D)DNA irradiation 120 min - AO-EB. [DNA]-2.8∙10-4 mol/L (P), [AO]-0.14∙10-4 mol/L, [EB]-0.14∙10-4 mol/L, [NaCl]-10-2 mol/L.
Additionally, temperature effect on FRET stability was investigated. We studied the effect of heat on DNA solution at various temperatures T=50, 60, 70, 80 and 90°C and, also, we investigated the boiling effect on DNA depending on the time of boiling [22]. The results are given in Table 2.
By analysing data given in Table 2, we can make a conclusion that the laser irradiation of AO, irradiation of Ar lamp, and the effect of heating decrease the concentration of undamaged areas of DNA double helix, i.e., the sites, which are able to intercalate dye molecules such as AO and EB. In its turn, it increases intercalator concentration per undamaged area of double helix, i.e., it decreases the distance between AO and EB and finally increases FRET effectiveness.
Glycoside linkage (C(I)-N(9)) hydrolysis in DNAdepurinization
The interaction of H+and Men+ with N(3) and N(7) of guanine in DNA with certain probability leads to, on the one hand, double proton transfer (DPT) [16] and on the other, to hydrolysis of glycosidic linkage C(1)-N(9).
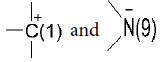
The reaction takes place with participation of dissociated molecules of H2O (OH– and H+). Of course, H+ approaches negatively charged N– as OH– goes to C+:
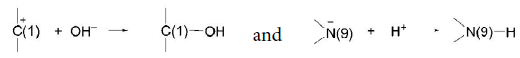
It needs to be mentioned that depurination can take place only in unwinded sites of DNA double helix. The dependence of the probability of unwinding of the central AU and GC base pairs in RNA double helix on the adjacent pairs was investigated in [23], and it was shown that the probability of opening of the G-C pair is minimum and equal to 0.3·10–5 in sequence:
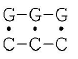
and the probability of opening of the A-U is maximum and equal to 120·10–5 in sequence:
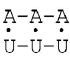
Phosphordiester-linkage hydrolysis in DNA
A hydrolysis reaction requires the presence of H+ and OH- ions the concentration of which, in water with a (natural for living cell), is 10 mol/L, i.e., the electrolytic dissociation constant is 10-14. Naturally, at such concentrations, the probability of hydrolysis is very small. In vivo phosphodiester link hydrolysis in DNA and RNA is carried out by nuclease ferments (DNase and RNase) and in vitro photo energy dissipation in solution acts as a catalyst first in phosphodiester and glycoside link hydrolysis reactions (Figure 8). It cannot be excluded that, in discoloration and degradation of aromatic compounds, the hydrolysis reaction is also important. As life-Time (τ) of exited AO singlet state is ~10-9 s (ττAO =1.5 × 10-9, τAO-DNA=5.2x 10-9[24], the possibility that a oxygen molecule ([O2]=1.3 × 10-6 mol/L at T=298K) can collide with an AO molecule is too small. At the same time, triplet excited state is unusual for AO molecule at room temperature [25,26].
So, in case of DNA complex with AO at photodynamic effect in solutions, the principal oxidant is not an oxygen molecule but H+ ions. It is also connected to the fact, which we have shown, that for H+ ions mobile-adsorption state of DNA is typical. It should be noted that, during the photodynamic effect, H+ local concentration depends on electrolytic dissociation of water molecules. So, at photon absorption by chromophore, a part of energy dissipates into heat as a result of conversion which we consider as energy transfer from electron vibration levels of organic molecules (in our case AO) to water molecule vibration levels (see IRET in Scheme 1) and it should increase the constant of electrolytic dissociation.
The energy of an absorbed photon is enough for 3 water molecules to undergo electrolytic dissociation. In the place where AO exited molecule undergoes total energy dissipation, we can get high local concentration of H+ and OH- ions in immediate proximity to DNA, and thus hydrolysis possibility of both phosphodiester and glycoside is significant. Phosphodiester and glycoside links can be presented as on Figure 8.
It is shown the application of the original nanoscale method of a laser induced FRET to a donor-acceptor intercalator pair for the quantitative and qualitative study of stability quality DNA double helix in a solution, in real time in the following biologically important processes: photoirradiation, photodynamic effect and electron excitation energy transfer in strongly scattering environment (colloidal) with multiple scattering of light, i.e., in processes that can be successfully used in light therapy of cancer, dermatology, wound healing etc.
The method of FRET shows that the use of non-equilibrium inductively coupled low-pressure plasma of low-capacity (some mW) as an optical source of line spectrum in near IR region of electromagnetic spectrum leads to the worsening of the quality of DNA double helix. This was interpreted as the hydrolysis of glycoside and phosphodiester bonds in DNA at the expense of exciting the large-amplitude overtones of the valent vibration of water molecules associated with DNA, which leads to the electrolytic dissociation of water.
It is shown that the photodynamic effect, which we consider as reradiation of the light from intercalator (AO) to the DNA (~10-20%) excites overtones of the valent vibration of water molecules associated with DNA, thereby activates their dissociation into H+ and OH- and this leads to the hydrolysis reaction of glicozide and phosphodiester bonds in DNA.
The authors express their gratitude to Prof. Nodar Tsintsadze, for the interest and permanent kind attention to our work. The authors also express their gratitude to Prof. Jamlet Monaselidze and Dr. Georgi Burdzhanadze for useful discussions and Ms. Ketevan Mdzinarishvili for her help in preparing the original manuscript.