Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Mini Review - (2021)Volume 12, Issue 7
The contemporary Fontan operation has revolutionised the management of complex congenital heart disease. Despite its unrivalled success, a substantial burden of disease persists in the growing adult Fontan population. There is accruing evidence to suggest that complications late after Fontan may reflect inadequate preparation during earlier staging operations. To this effect, there has been a paradigm shift towards strategies which aim to optimise the anatomy and physiology during the palliation process. The role of maintaining anterograde pulmonary blood flow has garnered substantial interest in recent years; however, despite an expanding evidence base, there is an absence of studies which attempt to correlate haemodynamic with clinical outcome data. This communication reports the findings of our recent retrospect cohort study and contextualises this within the growing body of literature. Looking to the future, randomised controlled trials remain highly unlikely; however, we propose solutions which monopolise on the expeditious advancements possible in today’s technological era, to affect further improvement in this area of unmet clinical need.
Heart disease; Paradigm shift; Haemodynamic; Cardiac MRI; Pulmonary artery
The Fontan operation represents a well-traversed surgical pathway for children with functionally Univentricular Hearts (fUVH) [1]. In an initial study assessing long-term outcomes, Francis Fontan quoted a 20-year survival of 65% in the best surgical candidates. However, adoption of contemporary surgical techniques has transformed the outlook of fUVH defects, with current 20-year survival rates range between 80%-90% [2,3], far exceeding Fontan’s initial prediction. Yet, despite incremental improvement in survival and impressive late functional outcomes, the contemporary Fontan remains far from the ideal solution for complex congenital heart disease.
Fundamentally, the Fontan circulation subjects the patient to a highly abnormal cardiopulmonary physiology. As the population of adult Fontan survivors grows, the pernicious effects of these haemodynamics become increasingly pronounced, with one pertinent study reporting a statistically significant decline in freedom from late adverse events with prolonged duration of follow-up [4]. It is clear that novel strategies are essential to minimise the inherent inefficiencies of a Total Cavo-Pulmonary Connection (TCPC) and overcome this almost expected gradual attrition: the ‘failing Fontan’ due to the Fontan paradox with associated complications of liver fibrosis, renal dysfunction and eventual cardio-pulmonary failure [5,6](Figure 1).
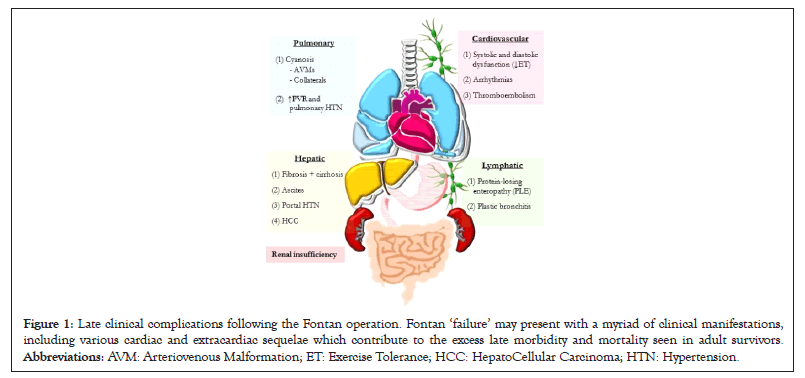
Figure 1: Late clinical complications following the Fontan operation. Fontan ‘failure’ may present with a myriad of clinical manifestations, including various cardiac and extracardiac sequelae which contribute to the excess late morbidity and mortality seen in adult survivors. Abbreviations: AVM: Arteriovenous Malformation; ET: Exercise Tolerance; HCC: HepatoCellular Carcinoma; HTN: Hypertension.
The role of maintaining Anterograde Pulmonary Blood Flow (APBF) during Fontan palliation has been garnering significant interest. With exclusion of right ventricular hydraulic energy, adequate cardiac output in the completed Fontan circuit, or TCPC, is contingent on low Pulmonary Vascular Resistance (PVR). Crucially, chronic privation of pulsatile pulmonary flow may be detrimental for endothelial function, capillary recruitment and pulmonary vascular development, all of which adversely impact PVR, as evidenced in animal models [7,8].
This premise has been corroborated in recent clinical studies; Chen, et al. [9] have shown favourable effects on haemodynamic proxies of endothelial function in patients with additional pulmonary blood flow, namely greater flow pulsation and region velocity, and, importantly, increased wall shear stress. Similarly, Ferns, et al. [10] have demonstrated important trophic effects (Nakata index) of residual pulsatile anterograde flow after BidirectionalGlenn (BDG), which persist long after Fontan completion.
We have also previously reported on the utility of preserving APBF after BDG [11]. However, there appears to be a scarcity of studies which correlate these findings with clinical outcome data. To this effect, our present study aimed to evaluate whether maintenance of APBF at the time of Fontan was associated with improved haemodynamic outcomes [12].Review of Literature
Study findings
Our cohort included 106 children who underwent Fontan completion at University Hospitals Bristol NHS Foundation Trust between1999 2018. 39 patients (36.8%) had APBF at the time of Fontan. Using routine preoperative cardiac catheterisation data, we calculated a novel pulmonary artery Pulsatility Index (PI) for each subject: PI=(pulmonary artery systolic pressure−pulmonary artery diastolic pressure)/mean pulmonary artery pressure. This formula was adapted from the doppler-derived blood velocity equation, which is commonplace in obstetric [13] and stroke medicine [14]. The study population was subsequently divided into thirds, according to PI magnitude, enabling both spectral and sub-group analysis. As expected, those with residual APBF have a significantly higher pulsatility index (Mann-Whitney U, p<0.0001).
There were no significant differences in early standard cardiac surgical outcomes, namely early mortality, post-operative ventilatory time and chest drainage time, duration of Paediatric Intensive Care Unit (PICU) and total hospital stay, or Systemic Arterial Oxygen Saturation (SaO2) at discharge, based upon pulsatility index.
Late outcome measures included change in ventricular function and atrioventricular valve regurgitation (echocardiography), exercise tolerance (Ross/NYHA), SaO2 at 1-year and last follow- up, medication support (diuretics and vasodilatory therapy) and intermediate (within one year) or late (after one year) Fontan failure. Whilst there was no statistically significant difference in long-term outcomes according to PI, there was a trend towards improved ventricular function in the medium PI sub-group (Kruskal-Wallis, p=0.0723). Similarly, there was a tendency towards higher SaO2 with increasing Pulsatility (Figure 2), but this did not each statistical significance on either spectral or subgroup analysis.
Figure 2: Trends in long-term systemic arterial oxygen saturation. The left-hand side depicts spectral analysis of SaO2 at (a) 1-year and (c) at last follow-up. The calculated Pearson R and associated p-value is shown. Data points in light blue indicate subjects with ‘low PI,’ with medium blue and dark blue representing those with ‘medium PI’ and ‘high PI’ respectively. On the right-hand sie, results from subgroup analysis are graphed, at (b) 1-year and (d) last follow-up, with associated p values (Kruskal–Wallis).
All patients in the high PI subgroup maintained SaO2 ≥ 90% at 1-year and last follow-up.
Our present study adds to an accruing body of literature attempting to delineate whether the proposed benefit of pulsatile pulmonary perfusion in the Fontan population translates from bench to bedside. Although auxiliary pulsatile APBF did not impact short- term outcomes or long-term prognosis in our cohort, there was a tendency toward improved postoperative SaO2, which may have lasting clinical benefits not identified within the time frame of our study. of note, all subjects in the ‘high PI’ subgroup maintained SaO2 ≥ 90% at both 1-year and last follow-up. Given that the chronic hypoxic environment prior to Fontan completion has previously been implicated as a contributor to the aberrant vascular function in this population [15], residual APBF may hold additional benefit in patients with lower pre-operative SaO2, including those with suboptimal preoperative pulmonary physiology as evidenced by higher PVR. Our present study was not designed to specifically evaluate this. We postulate that a further study is merited to investigate this relationship and answer these difficult questions.
There is a sound experimental evidence-base for preserving pulsatile pulmonary flow. On a molecular level, pulsatile shear stress upregulates endothelial Nitric Oxide Synthase (eNOS) and, through nitric oxide-mediated vasodilation, promotes vascular compliance [16,17]. This premise has been replicated in animal studies, including a chronic porcine model of BDG [18]. Here, micro-pulsatility (achieved by partial ligation of the pulmonary artery) aligned with significantly lower PVR, compared with total absence of pulsatility (complete pulmonary artery ligation).
Whilst these findings are compelling, the clinical decision whether to maintain or exclude APBF is more divisive. Proponents advocate that pulsatile perfusion during staging may be an underemployed strategy to protect and optimise the pulmonary vasculature: given the passive nature of cavo-pulmonary flow, even marginal increases in PVR can have profound consequences in the Fontan patient, with worsening preload deprivation and circulatory output [19]. However, opponents remain concerned that accessory pulmonary flow contributes to excessive ventricular volume loading, thereby mitigating some of the advantageous effect of the BDG [20]. It is evident that further research is required to elucidate whether there is an optimal level of auxiliary anterograde flow to retain adequate pulsatility to nurture the endothelium, without significantly loading the single ventricle. With the capacity to facilitate more robust pulmonary pulsatility quantification and more accurate ventricular and atrioventricular valve functional assessment, we posit that Cardiac MRI (CMR) may be a powerful tool for evaluating these parameters in future related studies.
The distinction between ‘pulsatile’ and ‘non-pulsatile’ flow is more ambiguous in human subjects as compared with animal models, adding a further layer of complexity. Although pulmonary artery banding should leave ‘pulsatile’ flow, where banding restricts the outflow tract to subcritical levels, this might closely emulate laminar blood flow. Equally, in the setting of systemic-to-pulmonary artery shunt, or aortopulmonary collateralisation, haemodynamic may be salutary and possess a pulsatile character, more closely replicating the arterial, as compared with a venous, waveform. From this, it is clear that novel non-invasive modalities to assess pulsatility could have great utility in investigating the translation of pulsatile perfusion to clinical practice, and ultimately help optimise clinical decision making in the palliation process.
Management of the failing Fontan represents a major clinical challenge. This review seeks to encourage clinicians to take a more proactive role during the initial staging steps, to identify children who may benefit from such optimisation approaches, rather than focusing solely on strategies which react to the failing circuit.
The idealistic approach going forward would be a recommendation for randomised controlled trials comparing pulsatile APBF versus total exclusion of additional pulmonary flow. This would ultimately provide robust end-point clinical data to justify one surgical strategy over the other. However, this is an immense oversimplification; first, given the heterogeneity of complex congenital heart disease, a ‘one-size-fits all’ approach is unlikely to yield success. Secondly, it is a fundamental prerequisite of clinical practice to closely consider the risks and benefits of any proposed action on a case-by-case basis, and to communicate this clearly to the patient and family to inform consent. As such, the ethical implications of randomisation to surgery in a paediatric population would at best be contentious, especially when considering that patients with certain fUVH pathologies would predictably respond poorly to a particular approach, aligning with poor outcomes. Thus, randomised controlled trials are highly unlikely in this arena.
From the recent study by Chen, et al. which employed computational fluid dynamic simulation, the benefits of APBF appear to be contingent on numerous measurable haemodynamic parameters. Whilst APBF was shown to be accompanied by an improvement in indexed energy loss and systemic arterial saturation, it also adversely impacted pressure in the right superior vena cava and subsequently increased heart load. With such technological advancements, it may become habitual and commonplace to measure these indices in clinical practice. Analysis of these parameters in large subgroups of fUVH patients, and correlating them with clinical outcome over longer time frames, may facilitate identification of specific defects which may respond more favourably to maintaining APBF. As such, we propose that efforts directed at national, and, preferably, international collaborations to formulate large datasets of haemodynamic data could be fruitful. This would ultimately enable creation of robust risk-stratification algorithms and pave the way for a future which promotes a personalised management approach.
Citation: Tulloh RMR, KaliaK (2021) The Promises of Pulmonary Pulsatilityâ??Should we Leave Forward Flow in the Fontan Operation? J Clin Exp Cardiolog.12:686.
Received: 27-May-2021 Accepted: 10-Jun-2021 Published: 17-Jun-2021 , DOI: 10.35248/2155-9880.21.12.686
Copyright: © 2021 Tulloh RMR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.