Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2017) Volume 6, Issue 2
Withania somnifera popularly known as ashwaganda or Indian Ginseng is a well known plant in India. Furthermore, it is a common plant in Egypt. The objective of this study is to evaluate the effectiveness of the extracts of different parts of the plant in the protection against oxidative stress and its therapeutic potential in the treatment of Alzheimer’s. All the tested extracts showed significant anticholinesterase activity, with the highest activity produced by the ripe fruit extract, significant nitric oxide scavenging activity, strong haemolysis protection as well as strong antioxidant activity. Unlike the Indian plant that is reputed for the medicinal use of its roots, the results of the present study also suggest that both the fruits and leaves of Egyptian plant have promising activities. Withanolide S isolated from its leaves extract, could be regarded as an interesting drug candidate for treatment of Alzheimer’s as it showed strong in vitro acetylcholinesterase (AChE) inhibitory activity with IC50 value of 0.00035 μM, strong haemolysis protection and antiinflammatory activities. Molecular docking studies was carried out in order to explore its exact mechanism of action.
Keywords: Withania somnifera; Ashwaganda; Alzheimer’s; Withanolide S; Aticholinesterase
Alzheimer’s disease (AD), the most common progressive form of dementia affecting middle-aged and elderly men and women occurs as a result of malfunctioning of different biochemical pathways especially the hyperactivation of acetylcholine esterase (AChE). Currently, some plant based constituents have been clinically used for AD symptomatic management for example Galanthamine and physostigmine [1].
Withania somnifera (Ashwgandha or Indian ginseng), is used in Ayurvedic medicine as an agent which promotes learning acquisition and memory retrieval. Its extract is used alone or in combination with other herbal drugs for the reversal of cognitive deficits associated with old age, chronic illness and behavioral disorders [2-4]. It contains bioactive compounds (withanolides and withanamides) which represent good candidates for alleviating Alzheimer’s. Withanolide A, withaferin-A, 2,3-dihydrowithaferin-A, withanoside IV and VI that were isolated from Withania root extract showed inhibitory effect towards AChE enzyme, and attenuated neuronal dysfunction by decreasing β-amyloid protein formation [5,6]. Withanolide A, has neuritic regeneration properties and facilitated the reconstruction of pre- and postsynaptic neurons, when neuron damage had already progressed [7]. Withanamides isolated from the fruit extracts especially withanamide A and C have been proved to be potent lipid peroxidation inhibitors and protect against β-amyloid formation [8,9].
To the best of our knowledge, few studies were carried on Egyptian W. somnifera focusing mainly on its cytotoxic activity [10,11].
This study was carried out to evaluate the pharmacological activities of crude extracts of different parts of W. somnifera and their major constituents relevant to the treatment of cognitive disorders including in-vitro cholinesterase inhibiting, antioxidant and anti-inflammatory effects.
Plant material
Withania somnifera (L.) Dunal plant was collected from Alexandria, Egypt in November 2014. The plant was kindly identified by Professor Atef M. Ibrahim, Department of Pomology, Faculty of Agriculture, Alexandria University, Egypt. A voucher specimen (WS105) has been deposited in the Pharmacognosy Department, Faculty of Pharmacy, Alexandria University, Egypt.
Isolation of Withanolide s
The dried powdered leaves (600 g) was defatted with light petroleum, the marc left was air dried then extracted with 75% ethanol (2 L). The ethanolic extract was filtered and the solvent was evaporated under reduced pressure to give a dark green semisolid residue (40 g). The residue was re-dissolved in 70% ethanol then extracted successively with light petroleum. The lower layer was concentrated and then partitioned between 60% ethanol and methylene chloride. The methylene chloride fraction (11.2 g) was chromatographed on a silica gel column. Elution was carried with methylene chloride with increasing concentrations of ethyl acetate till 100% ethyl acetate followed by increasing concentration of methanol in ethyl acetate till 100% methanol. The fraction eluted with 5% methanol in ethyl acetate (2 g) was purified on a silica gel column eluted with 25% ethyl acetate in n-hexane and purified using preparative TLC to isolate withanolide S (50 mg) in a pure form. It was used at different concentrations (0.2-1 mg/ml) as test materials for in vitro biochemical analysis.
Withanolide S(1): White needle shape crystals; UV λmax (MeOH) 225 nm. 1H- NMR (400 MHz, DMSO-d6): δ 5.6 (1H, dd, J = 10, 2 Hz, H-2), 6.5 (1H, ddd, J = 10, 5, 2 Hz, H-3), 1.90 (1H, dd, J = 19.1, 4.6 Hz, H-4a), 3.07 (1H, d, J = 19.1Hz, H-4b), 3.47 (1H, dd, J = 8, 4 Hz, H-6), 1.25 (1H, m, H-7a), 1.95 (1H, m, H-7b), 2.34 (1H, m, H-8), 1.98 (1H,td, J = 12, 5 Hz, H-9), 1.28 (1H, d, J = 14 Hz, H-11a), 2.12 (1H, dd, J = 14, 4.6 Hz, H-11b), 1.38 (1H, m, J = 12 Hz, H-12a), 1.57 (1H, td, J = 12 Hz, H-12b), 1.12 (1H, s, H-15a), 2.15 (1H, m, H-15b), 1.49 (1H,br d, J = 6 Hz, H-16a), 2.37 (1H,br d, J = 3 Hz, H-16b), 0.99 (3H, s, 18- Me), 1.12 (3H, s, 19-Me), 1.20 (3H, s, 21-Me), 4.6 (1H, dd, J = 13, 3 Hz, H-22), 2.3 (2H, m, H-23a,b), 1.73 (3H, s, 27-Me), 1.86 (3H, s, 28-Me). 13C -NMR (100 MHz, DMSO-d6): δ 204(C-1), 128.2 (C-2), 142.7 (C-3), 35.6 (C-4), 76.8 (C-5), 73.9 (C-6), 29.4 (C-7), 33.6 (C-8),34.1 (C-9), 51.8 (C-10), 22.7 (C-11), 32.4 (C-12), 54.7 (C-13), 83.1 (C-14), 31.2 (C-15), 36.3(C-16), 88 (C-17), 21.4 (18-Me), 15.6 (19-Me), 78.6 (C-20), 19.9 (21-Me), 81.9 (C-22), 34.9 (C-23), 151.4 (C-24), 120.7 (C-25), 166.6 (C-26), 12.6 (27-Me), 20.8 (28-Me). ESI-MS m/z 503 [M - H]-.
Preparation of extracts
Dried leaves, roots, ripe and unripe fruits of W. somnifera were powdered separately and 10 g of each powder was extracted with ethanol in 1:10 w/v ratio for 24 h. The filtered ethanolic extracts were concentrated under reduced pressure. The dried extracts were dissolved in dimethylsulfoxide (DMSO) and stock solutions of the extracts were appropriately diluted to get different concentrations (0.2-1 mg/ml) for each extract, and were used for biochemical analysis.
Chemicals
Acetylthiocholine iodide (ACTI), 5,5′-dithiobis[2-nitrobenzoic acid] (DTNB), diphenyl-1-picrylhydrazyl (DPPH) and Sulphanilamide were purchased from Sigma-Aldrich, USA. N-(1-Naphthyl)ethylenediamine dihydrochloride (NED) was purchased from Win Lab, UK. Sodium nitrite, Silica gel (230-400 mesh), precoated TLC plates silica gel 60 (GF-254) were purchased from Merck, Germany. All the solvents used were of analytical grade.
The 1D and 2D NMR analysis were obtained using a Bruker avance III (switzerland) 400 MHz. Residual peaks of the deuterated solvents were used to reference the spectrum.
ESI-MS spectra were measured on Waters Xevo G2-S Q-TOF mass spectrometer (Milford, MA, USA).
Preparation of brain homogenate
Six Balb/c mice were obtained from animal house of medical research institute, Alexandria University. After anesthesia, six brains were isolated and washed in cold saline, one gram of the brains was homogenized in 9 ml phosphate buffer saline. The homogenate was centrifuged at 3000 rpm and the supernatant was used for further biochemical estimations.
The experimental protocol was approved by the Animal Care & Use Committee (ACUC), Faculty of Pharmacy, Alexandria University, Egypt and was given number (AUC17/12).
Blood Sample
Fresh whole blood (3 ml) was kindly donated and collected in heparinized tubes to protect blood against coagulation.
Preparation of erythrocyte suspension
A volume of isotonic buffer solution (7.5 ml; 50 ml of 1M sodium phosphate buffer, pH 7.4 mixed with equal volume of NaCl (170 g/ml) then complete the volume to 1000 ml with distilled water) was added to 100 μl of fresh whole blood and then was centrifuged at 3000 rpm for 10 min. A volume of isotonic buffer solution equivalent to that of the supernatant was used to re-suspend the red blood pellets (the reconstituted red blood cells).
Acetylcholinesterase activity
Acetylcholinesterase (AChE) activity was assessed according to the method of Ellman et al in which, 130 μl phosphate buffer (0.1 M pH 7.4) were added to a mixture of 20 μl brain homogenate and 20 μl of different concentration of each extract (test) or DMSO (control), then incubated for 45 min at 37°C. Five μl of substrate ACTI (75 mM) were added, mixed well and incubated for 15 min at 37°C. Finally, 60 μl DTNB (0.32 mM) were added and left for 5 min. The absorbance was measured at 405 nm and the specific activity was calculated using the following equations.

Where, 1.36 × 104 is the molar extinction coefficient of DTNB.
Antioxidant assay
Determination of Dipheynl-α picrylhydrazyl (DPPH) radical scavenging activity
DPPH radical scavenging assay of the total extract was performed by using the previously established and modified methodology by Choi et al. Assays were performed in flat bottom polystyrene 96 well microtiter plates. To 100 μl of each sample (0.2-1 mg/ml) in DMSO, 25 μl DPPH (1 mM) in ethanol was added. The resultant mixture was briefly shaken and maintained at room temperature, in the dark for 30 min. At the end of this period, the absorbance (A) of the mixture was measured at 490 nm, using ELISA. Scavenging ratio of DPPH assay calculated as follows:
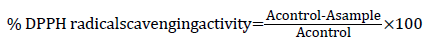
Antiinflammatory Activities
Nitric oxide scavenging activity: Nitric oxide scavenging activity was determined by the method described by Green et al, Sodium nitroprusside (5 mM) in phosphate buffered saline (pH 7.2), was mixed with different concentrations (0.2-1 mg/ ml) of the extracts and withanolide S then incubated at 25°C for 30 min. Samples from the above were reacted with Griess reagent (1% sulphanilamide, 2% phosphoric acids and 0.1% Naphthylethylenediamine dihydrochloride) for 150 min at room temperature. The absorbance of the chromophore (A) formed during the diazotization of nitrite with sulphanilamide and subsequent coupling with naphthylethylenediamine was read at 546 nm. The scavenged ratio of NO calculated as follows:
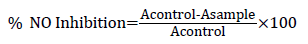
In vitro human red blood cell membrane stabilization method (HRBC)
The assay was determined by using the method of Shinde et al. The reconstituted red blood cells were used for the estimation of antiinflammatory property. Different concentrations of each extract and withanolide S (test) or DMSO (control) were separately mixed with 125 μl of isotonic buffer or 125 μl of distilled water to simulate hypotonicity-induced-inflammation and 50 μl of erythrocyte suspension. The mixtures were incubated at 37°C for 60 minutes then centrifuged at 1000 rpm for 10 minutes. The supernatant liquid was decanted and the hemoglobin content was estimated by measuring absorbance at 560 nm. The percentage haemolysis was estimated by assuming the haemolysis produced in the control as 100%. Haemolysis prevention and anti- inflammatory properties were calculated as follows:
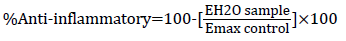
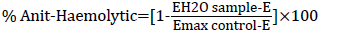
Where E is the sample absorbance in isotonic buffer, EH2O is the sample absorbance in water, and Emax is the control absorbance in water.
Docking studies
The protein structure protein data bank (PDB) code 1EVE, which is three-dimensional (3D) structure of the anti-Alzheimer drug, e2020 (Aricept®), complexed with its target AChE with a resolution of 2.50 Å was retrieved from Brookhaven PDB and used for the validation of the docking algorithm.
The three dimensional structure of withanolide S was derived by Avogadro software and the energy minimized compound was prepared. Cdt X-ray crystallographic structure of human BACE (3TPR Crystal structure of BACE1 complexed with an inhibitor) which was obtained from the RCSB Protein Data Bank. The accurate docking was performed using the docking tool iGEMDOCK v2.0. Based on the binding energy in kcal/mol. numbers of runs taken are 70 and max interactions were 2000 with population size 200 and with an energy threshold of 100 also at each step least ‘min’ torsions/translations/ rotations are tested and the one giving lowest energy is chosen. The hydrophobic preference and electrostatic preference were set to 1.00. The binding site of the target was identified at a distance 8 Å (Figure 1).
Statistical analysis
All results are presented as means of triplicate experiments. All experiments were conducted in triplicate and values expressed as mean ± standard deviation, correlations were carried out using the correlation and regression in the EXCEL program.
Chemistry
Figure 2 shows the structure of compound 1 isolated from leaves of W. somnifera . The molecular formula was established as C28H40O8 by analysis of the (ESI-MS) spectrum which showed [M - H]- peak at m/z 503. The 13C-NMR and 1H-NMR spectra showed the characteristic signals of steroidal Δ2-1-one system; a characteristic feature of withanolide skeleton. 1H and 13C-NMR spectra indicate clearly the presence of a 17 α -oriented side-chain where the values of rings C,D and the lactone side chain were similar to those of withanolide E [12], withanolide C [13], and withaperuvin [14].
The stereochemistry of the two hydroxyl groups at C-5, C-6 were assigned to be 5 α,6 β based on the following data; Resonances of hydrogens/carbons of ring A and B were in complete agreement to those of withanolide compounds, possessing the same configuration. The trans-fusion of the A/B ring system was confirmed by the 13C NMR spectrum [12].
The presence of a β -OH at C-5 causes an upfield shift of C[19] to around δ 8-9.6 ppm because of gauche-interaction and shielding of the C-19 resonance. However, no such upfield shift of C[19] was observed and its signal was observed at δ 15.6 that supports the 5α configuration of OH group. The molecular identity as withanolide S was confirmed by matching its spectral data with previous reports [12,15]. Assignments of all functional groups were achieved by 1H, 1H-COSY, HSQC and HMBC which further ascertained its identity.
Indian chemotypes are characterized mainly by the presence of 6α, 7α-epoxy withanolides with withanone and withanolide A representing the main components along with some 20-hydroxywithanolides like withaferin A. Besides, several sub chemotypes have been detected.
Phytochemical analysis of Egyptian plant suggested that it belongs to the Israeli chemotype III as we have isolated 5-ene withanolides, 17 α-oriented side chain withanolides and 20 hydroxy withanolide with the major withanolide being withanolide S (0.18%) in leaves (results under publication).
Previous study has proved that there is pharmacological difference between Italian chemotypes (equivalent to Israeli chemotype III) and the Indian one regarding the antioxidant activity [16].
To the best of our knowledge, this is the first investigation of this chemotype and of withanolide S concerning the protective effects against alzeheimer’s
Recent evidence indicates that the one molecule, multiple targets paradigm is effective for the treatment of complex diseases due to the drug's ability to interact with multiple targets responsible for the pathogenesis of the disease. Given the complex nature of AD and the fact that a single drug acting on a specific target (AChE) may have undesirable clinical effects, the “one molecule, multiple targets” paradigm strategy has also been applied in anti-AD drug design and was recently proven to be a successful strategy for the treatment of this multifaceted disease.
There are several strategies to tackle Alzheimer’s disease (AD), primarily, to augment cholinergic neurotransmission, which is fundamental to memory and learning processes [17], and have represented the primary approach in treating AD. Regarding AChE activity, the extracts of different parts of W. somnifera showed significant activity where the ripe fruits extract has strong activity when compared to Ginkgo biloba extract; a well-known cognitive enhancer; which shows IC50 of 0.268 mg/ml as reported in previous studies [18].
Oxidative stress and inflammation are the hallmarks for several diseases that affect mitochondria rich organs such as testicular tissue and brain. They increase in conditions such as Alzheimer’s disease (AD) and male infertility. Therefore, antioxidant and antiinflammatory activities are among pharmacological targets which are relevant to treatment of AD.
Reactive oxygen species play an important role in the AD neurodegeneration and pathogenesis; hence, the antioxidant capacities of plant extracts provide further support for their use in preventing the onset and progression of AD.
Different crude extracts of W. somnifera had moderate antioxidant activity when compared to Ginkgo biloba extract with IC50 of 0.04 mg/ml as previously reported [19].
Nitric oxide is a bio-regulator with crucial functions in human body as it is a key mediator in inflammation process. There is a strong relation between NO neurotoxicity and AD development as the reactive species NO- and peroxynitrite have been observed to cause neuronal damage in brains in AD [20,21].
Consequently, Nitric oxide scavenging activity of the extracts as shown in Table 1 has dual effects in management of AD, since the extracts act as antioxidant against reactive nitrogen species (RNS) and as antiinflammatory.
| Organ used | IC50 (mg/ml) | ||||
|---|---|---|---|---|---|
| % Inhibition of ACHE | DPPH Scavenging | NO Scavenging | Anti-Inflammatory | Anti-Haemolytic | |
| Root | 0.6 | 0.27 | 0.36 | 0.048 | 0.034 |
| Leaves | 0.75 | 0.26 | 0.17 | 0.063 | 0.058 |
| Unripe fruits | 0.5 | 0.23 | 0.18 | 0.042 | 0.04 |
| Ripe fruits | 0.17 | 0.25 | 0.19 | 0.108 | 0.071 |
| Withanolide S | 0.18 | 0.76 | 0.14 | 0.03 | 0.005 |
| Phyostigmine (positive control) |
2.8*10^-4 | ||||
| Diclofenac K (positive control) |
0.27 | 0.14 | |||
| Vit C (positive control) |
0.5 | 1.65 | |||
Table 1: IC50 for the biological activities of the extracts of different organs and withanolide S isolated from W.somnifera.
The inhibition of red blood cell membrane lysis by injurious substances was taken as a further measure of the mechanism of antiinflammatory activity [22]. The percentage inhibitions of haemolysis shown by all the extracts of W. somnifera were significant comparable to that obtained by diclofenac K.
Molecular docking analysis of withanolide s against proteins causing Alzheimer’s disease
The β -amyloid (Aβ ) peptide is the major constituent of amyloid plaques in Alzheimer's disease (AD) brain and is likely to play a central role in its pathogenesis. The β -secretase, β -site amyloid precursor protein cleaving enzyme (BACE1), is the enzyme responsible for initiating (Aβ) generation. Thus, BACE is a prime drug target for the therapeutic inhibition of (Aβ) production in AD.
Molecular docking simulation is a powerful and increasingly substantial tool for drug discovery, it can be used to model the interaction between a small molecule and a protein at the atomic level, which allow us to characterize the behaviour of small molecules in the binding site of target proteins as well as to elucidate fundamental biochemical processes.
As Withanolide S showed strong invitro acetylcholinesterase (AChE) inhibitory activity, molecular docking study was undertaken in order to understand its interaction with these target enzymes (Figure 3).
The structure of BACE1 site is generally composed of 11 pockets (also referred to as the “subsite”), i.e., S7, S6, S5, S4, S3, S2, S1, S1′, S2′, S3′ and S4′. The S1 pocket, hydrophobic in nature, is located at one of the entries of the cavity formed by the target protein interface, the S1′ and S2′ pockets are located at the other entry of the BACE1 active cavity. The S1′ pocket which is amphiphilic in nature is surrounded by two hydrophobic residues VAL326 and ILE22 as well as a few hydrophilic residues, such as THR329, LYS224, TYR198, ARG235, ASP228, THR72 and THR231. The S2′ pocket is amphiphilic in nature, formed by both hydrophilic residues such as SER35 and ARG128 and hydrophobic residues such as ILE126 and PRO70 [23].
The energy of binding of Withanolide S towards BACE1 was -95.7. TYR71, TYR198, ASP228, GLY230 and THR231 were identified as the binding sites.
withanolide S docking results showed hydrogen bonds with ASP32, ASP228 and THR232. Van der waal bonds with TYR71, TYR198, ASP228, GLY230 and THR231.
Docking with AChE
The 3D crystallographic structures of AChE revealed a deep and narrow gorge (20 Å) lined by conserved aromatic amino acids with two possible binding sites: the active site located at the bottom of the gorge and the peripheral site at the entrance. The active site comprises two sub-sites: the catalytic triad numbering: SER200, HIS440 and GLU327, which is essential for hydrolyzing the ester bond of ACh, and the ‘anionic site’ composed of aromatic residues, such as TRP84 and PHE330, that play an important role guiding the substrate towards the active site The second binding site is the peripheral site that lies at the entrance of the gorge and contains two principal amino acids, TRP279 and ASP72 [24].
The energy of binding of withanolide S towards 1eve was ‒124.5. TRP84, ASP72, TRY121, SER 122, TYR334, GLY118, TYR 121, TRP 279, PHE 330, PHE 331, TYR 334 were identified as interacting residues.
Withanolide S docking results showed hydrogen bonds between ASP72, TRY121, SER 122, TYR334 and TRP 84. Van der waal bonds with TRP84, GLY118, TYR 121, TRP 279, PHE 330, PHE 331, TYR 334.
All together the present study shows that the ethanolic extracts of the different parts of Egyptian W. somnifera , exhibited high acetylcholinesterase inhibitory effect as well as significant antioxidant activity, nitric oxide scavenging and anti-inflammatory activities [25,26]. Ripe fruit extract showed the best inhibitory activity towards AChE as well as good antioxidant and nitric oxide scavenging activities. Unlike the Indian plant that is reputed for the medicinal use of its roots, the results of the present study suggest that both the fruits and leaves of Egyptian plant have better biological activities than the roots [27,28].
Withanolide S which was isolated previously from the leaves of the Italian and Israelian plant [29,30], was isolated as a major constituent in the Egyptian plant. Withanolide S showed strong acetylcholinesterase (AChE) inhibitory activity so it could be regarded as an interesting drug candidate for treatment of Alzheimer’s disease. It also showed strong haemolysis protection and antiinflammatory activities. In addition, it was also observed to elicit significant BACE1 inhibition makes it interesting as potential dual inhibitor.
This work was supported in part by the University of Alexandria, Alexandria, Egypt.
No potential conflict of interest was reported by the authors.