Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Research Article - (2013) Volume 4, Issue 6
Objective: In the present study, the importance of the pulse pressure amplitude as a marker of the risk of myocardial infarction (MI) is discussed.
Methods: The 88 survivors of myocardial infarction were evaluated and a group of 106 healthy people is used as controls. The biomarkers: total cholesterol, HDL – cholesterol, serum triglycerides and LDL – cholesterol is measured with biochemical analyzer “Cobas Integra 400 (Roche)”. ANOVA analysis is performed and multivariate analyses are conducted with multiple logistic regression methods.
Results: The mean systolic blood pressure is significantly higher in MI participants. The relation between odds and pulse pressure amplitude, according to obtained models, indicates increasing of odds when the value of pulse pressure amplitude is greater. The p-value for overall model fit statistic is from 8.2173×10-5 to 0.0026 for different models and it is evidence that pulse pressure amplitude is a marker for MI risk. In the simplest model only pulse pressure amplitude is included as predictor variable whereas pulse pressure amplitude, sex and smoking are included in the most complex model. Results show that for a given pulse pressure amplitude odds of MI are significantly greater for men than for women. Expected increase of odds for the pulse pressure amplitude increase with 0.1 is obtained.
Conclusions: The studies prove that pulse pressure amplitude is a marker of myocardial infarction risk. When the pulse pressure amplitude is used for risk level evaluation at least sex should be taken into account. Obtained results show that increased pulse pressure amplitude is greater risk for men than for women.
Keywords: Myocardial infarction; Pulse pressure amplitude (PPA); Risk factors
Current guidelines for the diagnosis and management of hypertension have defined cardiovascular risk by the elevation of systolic blood pressure (SBP) and/or the elevation of diastolic blood pressure (DBP) [1-4]. However, the principal components of blood pressure consist of both a steady component (mean arterial pressure, MAP) and a pulsatile component (pulse pressure, PP) [4].
Pulse pressure (PP) is defined as the difference between systolic blood pressure (SBP) and diastolic blood pressure (DBP). Physiologically, both pressures increase throughout life due to the increase of stroke volume and/or peripheral vascular resistance (PVR). In the sixth decade of age, the PP increases with increasing SBP and decreasing DBP due to an increase of arterial stiffness [5,6].
In clinical practice there are some special situations when wide pulse pressure is an important diagnostic sign. Wide pulse pressure above 80 mmHg is an important character in severe aortic regurgitation [6,7]. A patent ductus arteriosus is widely considered as a difficult case when the patient shows wide pulse pressure and Corrigan´s pulse. The same applies to the aorto-pulmonary window and the proximal arterio venous fistulae [8].
Pulse pressure is considered as therapeutic target. Antihypertensive drugs have a different effect on compliance and central blood pressure, although the effect on peripheral blood pressure is equal [6,9].
Etiologic factors known to increase PP and pulse wave velocity include a reduction of elastic fibers, which are replaced by collagen [10], endothelial dysfunction and an increased expression of vasoconstriction substances (angiotensin II, endotelin, tromboxan) and decrease of vasodilatation substances (NO, bradykinin). In the literature, higher PP was related to smoking, diabetes mellitus [6,11], dyslipidemia [6,12], hyperhomocysteinemia [6,13], obesity and power sports activity.
An ejection of blood into the aorta generates a pressure wave (primary wave) that travels along the whole arterial vascular tree. A reflected wave (secondary wave) that travels backwards to the ascending aorta is principally generated in the small peripheral resistance arterioles. With increasing of arterial stiffness both the forward and the reflected waves propagate more rapidly along the vessels. Consequently, instead of reaching back the aorta during the diastole, the reflected pulse wave reaches it during the systole. The result leads to an increase of aortic pressure during systole and reduced pressure during diastole; this phenomenon is called PP amplification.
The relationship between brachial PP and height suggests that the phenomenon of peripheral pressure amplification is also affected by transmission length [14]. The increased left ventricle mass induced by the augmented afterload requires an increased oxygen supply. Therefore, a mismatch between oxygen demand and supply may occur, leading to myocardial ischemia, left ventricle diastolic and later systolic dysfunction. With decrease of DBP, the flow in coronary arteries also decreases [15]. Pressure amplification expressed by peripheral/ central pulse pressure ratio was shown to be linearly related to age (r= 0.7; p<0.001), with inverse linear relation to diastolic pressure in the younger group (r = 0.3; p<0.001) but not in older subjects [6,16].
The normal range of PP is not known. In a study of hypertensive subjects, those with PP 60 mmHg had higher values of left ventricle mass than those with PP 60 mmHg, despite similar mean pressures [17]. Vaccarino et al. reported that the increase of PP about 10 mmHg increases a heart failure risk about 14 %, coronary artery disease about 12 %, and all cause mortality about 6 % in population older than 65 years [18]. The NHANES I study showed that an increase of PP about every 10 mmHg increases cardiovascular death risk about 26% in the age 25-45, and about 10 % in the age 46-77 [19]. As the prospective study of Franklin demonstrated the prognostic significance of systolic, diastolic and pulse pressure varies with age.
In the present study, the importance of the pulse pressure amplitude (PPA) as a marker of the risk of myocardial infarction is discussed. The pulse pressure amplitude is dependent on the pulse pressure and systolic blood pressure.
In the present study 88 survivors of myocardial infarction were evaluated and a group of 106 healthy people was used as controls.
Systolic and diastolic blood pressure, weight and BMI were measured. Blood pressure measurements were performed in a hospital. Pulse pressure amplitude was determined by the formula 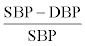 .The biomarkers: total cholesterol, HDL–cholesterol, serum triglycerides and LDL–cholesterol were measured with biochemical analyzer “Cobas Integra 400 (Roche)”. Questionnaire for clinically healthy individuals and questionnaire for survivors of myocardial infarction were developed.
.The biomarkers: total cholesterol, HDL–cholesterol, serum triglycerides and LDL–cholesterol were measured with biochemical analyzer “Cobas Integra 400 (Roche)”. Questionnaire for clinically healthy individuals and questionnaire for survivors of myocardial infarction were developed.
Data on some clinical characteristic are presented as means ± standard deviation (SD). ANOVA analysis was performed on a variety of factors examined by splitting the participants into different groups. First the survivors and controls were divided by sex. Then four groups: men experienced MI, healthy men, women experienced MI and healthy women were analyzed. The second separation was done by dividing the survivors and controls into smokers and no smokers. Then four groups: smokers experienced MI, healthy smokers, no smokers experienced MI and healthy no smokers were analyzed. Multivariate analyses were conducted with multiple logistic regression methods, and estimations of myocardial infarction risk were done.
Some clinical characteristic of participants with myocardial infarction and controls were summarized (Table 1). The mean systolic blood pressure was significantly higher in MI participants. The mean diastolic blood pressure was higher, but not significantly, in MI participants. The percent of smokers was slightly higher in controls and the percent of men was significantly higher in MI participants.
| Characteristic | Premature MI (n=88) | Controls (n=106) |
|---|---|---|
| DBP [mm Hg] | 83.86 ± 5.45 | 80.58 ± 9.98 |
| SBP [mm Hg] | 135.45 ± 9.96 | 125.36 ± 16.07 |
| Body weight [kg] | 82.69 ± 16.32 | 76.9 ± 17.58 |
| Height [cm] | 172.16 ± 8.15 | 168.31 ± 9.05 |
| BMI [kg/m2] | 27.92 ± 5.32 | 27.06 ± 5.62 |
| Total cholesterol [mmol/l] | 6.02 ± 0.86 | 5.31 ± 1.48 |
| Triglycerides [mmol/l] | 2.12 ± 0.77 | 1.62 ± 1.02 |
| HDL cholesterol [mmol/l] | 1.43 ± 0.62 | 1.37 ± 0.44 |
| LDL cholesterol [mmol/l] | 4.17 ± 0.99 | 3.44 ± 1.26 |
| Men / Women | 51/37 | 42/64 |
| Smoker (%) | 39 (44.32) | 51 (48.11) |
Data are presented as number (%) of patients or mean ± SD. SD – standard deviation; DBP – diastolic blood pressure; SBP – systolic blood pressure; BMI – body mass index; HDL – high–density lipoprotein; LDL – low–density lipoprotein; MI – myocardial infarction.
Table 1: Clinical characteristics of study participants.
The box plots for 1-way ANOVA test of PPA (Figure 1) did not indicate significant differences between four groups: men with MI; men without MI; women with MI and women without MI. The ANOVA F-statistic was 4.1965 with p-value 0.0067 and the hypothesis that all 4 of these groups’ means were equal had to be rejected. The multiple comparison test showed that the means of groups women with and without MI were significantly different (Table 2). Although there were not other statistically significantly different pairs of means some differences between group means were observed especially between groups: men without MI and women without MI.
| First group | Second group | Lower boundary of the CI | Difference between means | Upper boundary of the CI |
|---|---|---|---|---|
| Men with MI | Men without MI | -0.0219 | 0.0100 | 0.0418 |
| Men with MI | Women with MI | -0.0403 | -0.0073 | 0.0257 |
| Men without MI | Women without MI | -0.0092 | 0.0212 | 0.0515 |
| Women with MI | Women without MI | 0.0069 | 0.0384 | 0.0700 |
MI - myocardial infarction; CI–95% confidence interval
Table 2: Table of multiple comparison tests for men and women with and without MI.
The 1-way ANOVA test of PPA was done when separation of smokers and no smokers was used (Figure 2). The results did not denote significant differences between smokers and no smokers. Obtained F-statistic was 3.2719 with p-value 0.0223 and the hypothesis that all 4 of these groups’ means were equal had to be rejected. The multiple comparison test showed that the means of groups no smokers with and without MI were significantly different (Table 3). The differences in means of groups when separation of smokers and no smokers was used were smaller in comparison with separation of men and women.
| First group | Second group | Lower boundary of the CI | Difference between means | Upper boundary of the CI |
|---|---|---|---|---|
| S with MI | S without MI | -0.0136 | 0.0192 | 0.0519 |
| S with MI | NS with MI | -0.0349 | -0.0019 | 0.0312 |
| S without MI | NS without MI | -0.0191 | 0.0108 | 0.0408 |
| NS with MI | NS without MI | 0.0016 | 0.0319 | 0.0621 |
S–smokers; NS–no smokers; MI–myocardial infarction; CI–95% confidence interval
Table 3: Table of multiple comparison test for smokers and no smokers with and without MI.
Multiple logistic regressions were used to determine odds of MI. First a model with PPA as predictor variable was studied. The relation between odds and PPA, according to obtained model, indicated increasing of odds when the value of PPA was greater (Figure 3). The p-value for overall model fit statistic was 0.0026 and it was evidence that PPA was a marker of MI risk. The value of odds 1 took place when PPA was 0.3912. The results showed that for increase of PPA with 0.1 it was expected about 2.1397 times increase in the odds of MI.
In the second model PPA and smoking were included as predictor variables (1 for smokers and 0 for no smokers). The study of model showed that for a given value of PPA the odds are slightly greater for no smokers than for smokers (Figure 4). The value of odds 1 took place when PPA was 0.3801 for smokers and 0.4038 for no smokers. The p-values of PPA and smoking regression coefficients where respectively 0.0040 and 0.5418 and indicated that smoking did not contribute significantly to the prediction of MI. The results showed that for increase of PPA with 0.1 it was expected about 2.1448 times increase in the odds of MI. The p-value for overall model fit statistic was 0.0021 which is smaller than for the first model.
In the third model PPA and sex were included as predictor variables (1 for men and 0 for women). The study of model showed that for a given value of PPA the odds are greater for men than for women (Figure 5). The value of odds 1 took place when PPA was 0.4387 for women and 0.3442 for men. The p-values of PPA and sex regression coefficients where respectively 0.0070 and 0.0210 and indicated that both variables contribute significantly to the prediction of MI. The results showed that for increase of PPA with 0.1 it was expected about 2.0772 times increase in the odds of MI. The p-value for overall model fit statistic was 1.4237×10-4 which is smaller than for the first and the second models.
In the fourth model PPA, sex and smoking were included as predictor variables. The study of model showed that for a given value of PPA the odds are greatest for no smoking men and smallest for smoking women (Figure 6). The value of odds 1 took place when PPA was 0.3208 for no smoking men, 0.3633 for smoking men, 0.4225 for no smoking women and 0.4649 for smoking women. The p-values of PPA, sex and smoking regression coefficients where respectively 0.0066, 0.0147 and 0.3102 and indicated that the first both of variables contribute significantly to the prediction of MI. The results showed that for increase of PPA with 0.1 it was expected about 2.0827 times increase in the odds of MI. The p-value for overall model fit statistic was 8.2173×10-5 which showed that this model was the best.
The studies proved that pulse pressure amplitude was a marker of myocardial infarction risk. When the pulse pressure amplitude is used for risk level evaluation at least sex should be taken into account. Obtained results showed that increased pulse pressure amplitude is greater risk for men than for women. The obtained models could be used in clinical practice for prophylaxis of MI. The pulse pressure amplitude could be a therapeutic target.