
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2023)Volume 6, Issue 2
In the present study, Conformational analysis of 3-cyanophenylboronic Acid(3-CyBA) molecule have been carried by calculating Potential Energy Surface (PES) as a function of two dihedral angles, C1-B-O1-H and C1-B-O2-H, using DFT/B3LYP/6-31G (d) level of theory. As a result of PES, molecular conformers corresponding to low energy of title molecule, anti-syn, syn-anti, syn-syn, anti-anti, respectively, have been determined according to the orientations of the hydroxyl groups attached to the boron atom. The geometries of anti-syn, syn-anti, syn-syn, anti-anti, conformers of studied molecule were fully optimized at the Hartree-Fock (HF) and DFT/B3LYP levels of the theory with 6-311++G (d,p) basis set and compared with its crystal structure in the literature. The vibrational frequencies, infrared (FT-IR) intensities Raman (FT-Raman) scattering activities of all the conformers of the title molecule were calculated both methods and vibrational assignments were performed by means of Potential Energy Distribution (PED). Also, frontier molecular orbitals, the linear and nonlinear optics parameters, such as the polarizability (α) ground state dipole moment (μ) and the first-order hyperpolarizability (β) of 3-CyBA molecule, were calculated the same methods. The anti-syn conformer is found to be more stable than the syn-anti, syn-syn and anti-anti-conformers by 0.227, 1.078 and 4.577 kcal/mol in HF/6-311++G(d,p) and 0.248, 1.465 and 3.855 kcal/mol in DFT/B3LYP/6-311++G(d,p) level of theory, respectively. UV-visible absorption spectra such as excitation energies, absorption wavelengths (λ) and oscillator power (f) and stimulation contributions of all examined conformers were examined using TD-DFT/B3LYP and TD-HF methods and transitions were determined.
Conformational analysis; 3-cyanophenylboronic acid; Polarizability; Hyperpolarizability; Vibrational frequencies; UV-visible
Boronic acids show soft Lewis acids due to the empty orbital of the boron atom. This property makes boronic acids receptor, organic solar cells, protecting group and biologically attractive [1-4]. Boronic acids have many use areas in medicine, chemistry, biology and materials science. Boronic acids do not cause any environmental damage as they decompose to ortho boric acid and do not harm human health since they are non-toxic. In the systems that using liquid membrane system and allowing fructose to separate from other sugar mixtures have been tested many new many new anions, cations, borates and boronic acid derivatives that can form complexes with sugars [5]. Boronic acids have a special place in the design of chemical sensors used to detect the glucose molecule [6,7]. Boronic acid-containing polymers are also used in many biomedical applications such as HIV treatment to obesity, diabetes and cancer treatment [8]. In the literature, boronic acid and its derivatives have a special place in the definition and separation of carbohydrates [9]. Boronic acids play an active role in the identification of many biomolecules such as enzymes, proteins and antibodies, especially glucose for analytical and medical purposes and in the detection of diseases, as they can form stable and reversible diol complexes [10]. Boronic acids are molecules that can selectively bind carbohydrates using molecular suppression techniques [11]. Also, due to ability baryonic acid to form hydrogen bonding in the molecular complexes and coordination in metal complexes, these compounds continue to be of increasing interest and research. It has been known since 1953 that phenylboronic acids can be condensed with polyols and form boranate esters [12,13].
Theoretical chemistry provides convenience to those dealing with chemistry. With these programs, molecules can be observed from different angles by rotating them on the computer screen, their isomers and geometric structures can be understood, and their energies can be calculated. Ultraviolet (UV), Infrared Radiation (IR), Nuclear Magnetic Resonance (NMR) spectra can be drawn and even Molecular Orbital (MO) diagrams can be accessed [14-18]. In addition to experimental methods, computational chemistry methods are also used in the determination of corrosion inhibitors. The activity of an inhibitor, several quantum chemical parameters, can be calculated theoretically without the need for experimentation. The quantum chemical parameters generally used in theoretical studies on corrosion are atomic charges and molecular orbital energies [19-23].
The several studies have been carried out experimentally and computationally on the substituted penylboronic acid derivatives. The studies about crystal and molecular structure of phenylboronic acid and its ortho-, meta-, para-halogen or alkoxy groups and 3-amino-, and 3-cyano derivatives [24-30]. The investigation on supramolecular synthesis of phenylboronic derivatives were studied [17,21-23]. The study on conformational analysis of phenylboronic derivatives was reported. In this study, 3-cyanophenylboronic acid molecule, having the B(OH)2 and C≡N groups is modeled theoretical and molecular properties such as structural and electronic properties have been investigated using Hartree-Fock (HF) and Density Functional Theory (DFT) with B3LYP (Becke 3 Parameter Lee-Yang-Parr) model using the 6-311++(d,p) basis set in gas phase [14,31,32]. The search of literature on 3-CyBA revealed that its crystal and molecular structure of reported, but conformational analysis, electronic, linear and nonlinear properties of this molecule has not been calculated experimentally and theoretically. The potential energy surface of studied compound has been carried out by calculating 3D conformation analysis. Also, electron affinity A and ionization energy IP were defined as A=-ELUMO and IP=-EHOMO and by using these values have obtained global chemical reactivity ike chemical hardness (η), softness (σ), electronegativity (χ), electronic chemical potential (μ), and electrophilicity Index (ω). The molecular structure using numbering scheme of 3-CyBA is given in Figure 1.
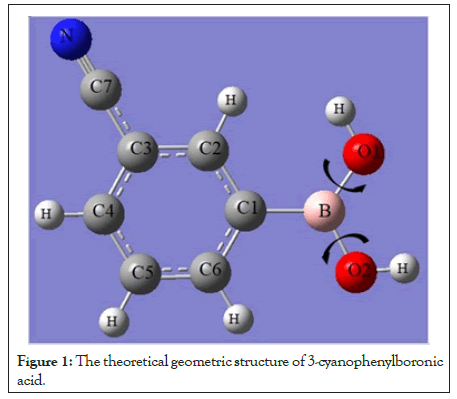
Figure 1: The theoretical geometric structure of 3-cyanophenylboronic acid.
Computational details
Firstly, quantum mechanical methods on the 3-CyBA molecule was performed by the aid of Gaussian 09W program package and Gauss view 5.0 molecular visualization programs in the gas phase. The conformational analysis of 3-CyBA molecule have been carried by calculating potential energy surface as a function of two dihedral angles, C1-B-O1-H and C1-B-O2-H, using DF/B3LYP/6-31G level of theory. As a result of PES, molecular conformers corresponding to low energy of title molecule, anti-syn, syn-anti, syn-syn, anti-anti, respectively, have been determined according to the orientations of the hydroxyl groups attached to the boron atom [34]. The geometries of anti-syn, syn-anti, syn-syn, anti-anti, conformers of studied molecule were fully optimized at the Hartree-Fock (HF) and Density Functional Theory (DFT) with B3LYP with 6-311++G(d,p) basis set. After optimization, structural parameters, vibrational frequency, the electronic energy, the dipole moment (μ), the Highest Occupied Molecular Orbital (HOMO) energy, the Lowest Unoccupied Molecular Orbital (LUMO) energy, the polarizability (α) and hyperpolarizability (β) of all the conformer of stuied molecule were calculated at HF/6-311++G(d,p) and DFT/B3LYP/6-311++G(d,p) level of theory in gas phase. The obtained vibrational wave numbers were scaled with appropriate scale factors and the assigning of these vibrational wavenumbers was made according to the potential energy distribution using the VEDA 4f program.
Conformational analysis
Conformation analysis of title molecule has been carried out by calculating three Dimensional (3D) potential energies of studied molecule. The Potential Energy Surface (PES) scan have been carried out as a function of two dihedral angles, C1-B-O1-H and C1-B-O2-H, varied between 0 and 360°C with increments of 10°C at B3LYP/6-31G (d) level of theory. By using data obtained from PES scan calculation, PES and potential energy contour format of 3-CyBA molecule have been plotted and are given Figures 2a and 2b. As seen in the Figure 2a, the potential energy changes of the different spatial orientations of the hydroxyl groups attached to the boron atom have been shown on the potential energy surface and potential energy contour format. It is seen that there is more than one conformation with maximum and minimum energy on the PES and potential energy contour. The points corresponding to the maximum and minimum energy conformations are numbered 1 to 13. The number 1 conformation has the lowest energy and corresponds to the anti-syn conformer. Conformations 2, 3, 4 and 5 correspond to the syn-anti conformer. Conformations 6 and 7 correspond to the syn-syn conformation. Conformations 8 and 9 correspond to the anti-anti conformation. The conformations 11, 12, 13 and 14 correspond to the maximum energy conformations. As a result of conformational analysis, molecular conformers corresponding to low energy of title molecule, anti-syn, four syn-anti, two syn-syn and two anti-anti have been determined according to the orientations of the hydroxyl groups attached to the boron atom, respectively. According to results of conformational analysis, for conformers with low energy such as anti-syn, syn-anti, syn-syn, anti-anti, both hydrogen atoms bonded oxygen are almost in the O1-B-O2 plane but in the conformation 11, 12, 13 and 14 corresponding to the maximum energy, these hydrogens are out of O1-B-O2 plane.
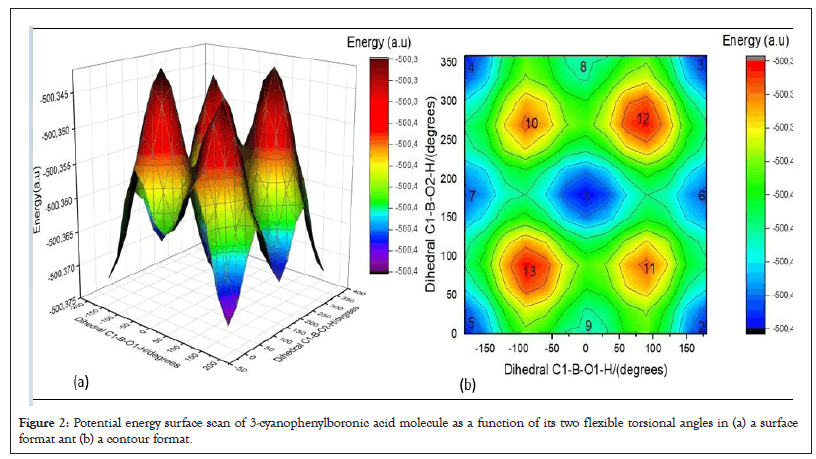
Figure 2: Potential energy surface scan of 3-cyanophenylboronic acid molecule as a function of its two flexible torsional angles in (a) a surface format ant (b) a contour format.
Geometrical structure
3-CyBA molecule, C7H6O2BN, is substituted benzene with two different functional grous a nitrile (−C≡N) and boronic acid (-B(OH)2). The all metastable conformatoin, an anti-syn, four syn-anti, two syn-syn, two anti-anti, on the PES of studied molecule were optimized at the HF/6-311++G(d,p) and DFT/B3LYP/6-311++G(d,p) levels of the theory. It has been observed that when more than one metastable conformation with the same name is optimized, these conformations convert to a stable conformer with the same name and the stable conformers determined as a result of optimization, anti-syn, syn-anti, syn-syn and anti-anti is given in Figure 2b. The stability of the optimized structures of all the conformers has been affirmed by vibration modes calculation, which gave positive values for entire vibration modes (Figure 3).
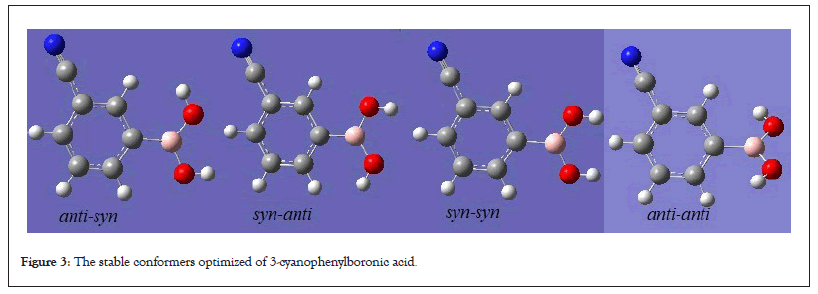
Figure 3: The stable conformers optimized of 3-cyanophenylboronic acid.
Afterward, structure parameter of stable conformers as an initial geometry utilized to compute polarizability, hyperpolarizability, HOMO, LUMO energies calculated HF/6-311++G(d,p) and DFT/B3LYP/6-311++G(d,p) levels are given Table 1. The energy band gap was obtained using HOMO and LUMO energies (ΔE=ELUMO-EHOMO, where ELUMO and EHOMO are HOMO and LUMO energies). While the band gap values of the three conformers calculated DFT/B3LYP/6-311++G(d,p) levels of the theory have the same value (5.66 eV), that of the anti-anti is close to this value. This result shows that the energy of the molecule is not affected by conformers. The calculated energy band gap on phenylboronic acid derivatives in the literature have been determined as 5.39 eV for 4-(methoxycarbonyl)-phenylboronic acid and 4.97-5.96 eV.
| B3LYP/6-311++G(d,p) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Electronic energy | ΔE | μ | α | β | EHOMO | ELUMO | Eg | |||
| (a.u) | (kcal/mol) | (D) | (a.u) | (a.u) | (a.u) | (a.u) | (eV) | |||
| a-s | -500.661669 | 0 | 3.55 | 105.75 | 52.44 | -0.281065 | -0.073087 | 5.66 | ||
| s-a | -500.661275 | 0.248 | 5.69 | 105.78 | 60.73 | -0.281785 | -0.073719 | 5.66 | ||
| s-s | -500.659334 | 1.465 | 6.5 | 106.37 | 125.89 | -0.27053 | -0.062582 | 5.66 | ||
| a-a | -500.655525 | 3.855 | 4.07 | 105.61 | 63.1 | -0.288744 | -0.079446 | 5.7 | ||
| HF/6-311++G(d,p) | ||||||||||
| a-s | -497.687644 | 0 | 3.63 | 95.6 | 23.59 | -0.362239 | 0.033789 | 10.78 | ||
| s-a | -497.687282 | 0.227 | 5.94 | 95.64 | 46.96 | -0.363826 | 0.028076 | 10.66 | ||
| s-s | -497.685925 | 1.078 | 6.7 | 96.07 | 39.22 | -0.353329 | 0.026112 | 10.32 | ||
| a-a | -497.680349 | 4.577 | 4.24 | 95.55 | 41.7 | -0.371474 | 0.027715 | 10.86 | ||
Table 1: HOMO, LUMO energy, dipole moment, polarizability, hyperpolarizability, and energy gap (ΔE) of the compound (ΔE/kcal mol-1).
The importance of polarizability and initial hyperpolarizability in molecular systems is associated with electronic communication between donor and acceptor groups due to intramolecular charge transfer [35]. The molecular electronic dipole moment, molecular polarizability, polarizability anisotropy and molecular initial hyperpolarizability of conformers (anti-syn, syn-anti, syn-syn and anti-anti) were calculated using the same methods and the results are given in Table 1.
The optimized structural parameters of these stable conformers along with the experimental parameters in the literature are listed in Table 1 [30]. As can be seen from Table 1, according to results of conformational analysis, for conformers with low energy such as anti-syn, syn-anti, syn-syn, anti-anti, both hydrogen atoms bonded oxygen are almost in the O1-B-O2 plane and anti-syn, syn-anti, syn-syn conformers are planar but anti-anti conformers are not planar that is, conformational preference of dihedral angle of four possible conformers between the boronic acid group and benzene ring have varied between 0° at the both methods and -35.61°. For anti-syn, syn-anti and syn-syn conformers, this angle has been calculated as 0° but anti-anti-conformer is -35.61° (B3LYP) and -35.05° (HF). As can be seen from Table 2, B-C bond lengths are in the range of 1.5651-1581 Å (B3LYP) and 1.570-1.589 Å (HF). For phenylboronic acids with the type and position of the substituents on phenyl ring, these values are 1.562 Å, 1.5747 Å and 1.5675 Å experimentally, 1.572-1586 Å, 1.565-1598 Å and 1.177 Å theoretically [9,24,30,36-38]. In general, B-O bond length in the unsubstituted phenyl boronic acids can change according to orientation of the hydrogen atoms attached the oxygen atoms to phenyl ring. B-O1/2 bond length is in the range of 1.5651-1581 Å (B3LYP) and 1.570-1.589 Å (HF). According to published articles, experimentally B-O1/2 bond length have been reported as 1.3455/1.3661 Å, 1.354/1.369 Å these values have been calculated theoretically as 1.363/1.367, 1.363/1.373 Å (B3LYP) and 1.5324/1.3661 Å (HF) [15].
| Bond length (Å) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B3LYP/6-311++G(d,p) | HF/6-311++G(d,p) | |||||||||||||
| Atoms | Expa | a-s | s-a | s-s | a-a | a-s | s-a | s-s | a-a | |||||
| C1-C2 | 1.3917(17) | 1.3987 | 1.4012 | 1.3998 | 1.4005 | 1.3901 | 1.3935 | 1.3915 | 1.3918 | |||||
| C1-C6 | 1.3987(18) | 1.4056 | 1.4031 | 1.4038 | 1.4052 | 1.3965 | 1.3933 | 1.3941 | 1.3955 | |||||
| C1-B | 1.5747(18) | 1.5718 | 1.5718 | 1.5651 | 1.5812 | 1.5786 | 1.5787 | 1.5704 | 1.5892 | |||||
| C2-C3 | 1.3948(17) | 1.4015 | 1.4002 | 1.4008 | 1.4017 | 1.389 | 1.3872 | 1.3881 | 1.3888 | |||||
| C3-C4 | 1.3882(19) | 1.4019 | 1.4029 | 1.4031 | 1.4018 | 1.3886 | 1.3904 | 1.3904 | 1.3887 | |||||
| C3-C7 | 1.4401(18) | 1.4316 | 1.4324 | 1.4321 | 1.432 | 1.4429 | 1.4436 | 1.4433 | 1.4434 | |||||
| C4-C5 | 1.3825(19) | 1.3905 | 1.3894 | 1.3901 | 1.3899 | 1.3827 | 1.3811 | 1.382 | 1.3823 | |||||
| C5-C6 | 1.3834(91) | 1.3927 | 1.3936 | 1.3935 | 1.3935 | 1.3847 | 1.3861 | 1.386 | 1.3852 | |||||
| C7-N | 1.1416(18) | 1.1557 | 1.1555 | 1.1558 | 1.1555 | 1.1311 | 1.1309 | 1.1313 | 1.1308 | |||||
| B-O1 | 1.3661(18) | 1.3704 | 1.3642 | 1.3708 | 1.3646 | 1.3575 | 1.3512 | 1.3578 | 1.3507 | |||||
| B-O2 | 1.3455(17) | 1.3653 | 1.3712 | 1.3717 | 1.3654 | 1.3522 | 1.3579 | 1.3585 | 1.3514 | |||||
| Bond angle (°) | ||||||||||||||
| C2-C1-C6 | 117.51(11) | 117.78 | 117.8 | 118.32 | 117.62 | 117.7 | 117.71 | 118.24 | 117.58 | |||||
| C2-C1-B | 122.70(11) | 122.47 | 119.36 | 120.64 | 121.05 | 122.74 | 119.18 | 120.65 | 121.09 | |||||
| C6-C1-B | 119.79(11) | 119.75 | 122.85 | 121.05 | 121.32 | 119.56 | 123.1 | 121.11 | 121.33 | |||||
| C2-C3-C4 | 121.02(12) | 119.87 | 120.1 | 120.03 | 120.02 | 121.07 | 120.81 | 120.61 | 121 | |||||
| C2-C3-C7 | 119.00(21) | 119.95 | 120.1 | 120.09 | 119.97 | 120.28 | 120.47 | 120.37 | 120.43 | |||||
| C4-C3-C7 | 119.98(11) | 120.17 | 119.8 | 119.88 | 120.01 | 119.75 | 119.93 | 119.93 | 119.76 | |||||
| C1-B-O1 | 123.75(11) | 124.7 | 117.93 | 117.93 | 122.43 | 119.98 | 119.6 | 119.69 | 119.8 | |||||
| C1-B-O2 | 117.10(12) | 117.79 | 124.43 | 117.86 | 122.18 | 124.46 | 117.88 | 118.19 | 122.01 | |||||
| O1-B-O2 | 119.15(12) | 117.51 | 117.64 | 124.21 | 115.38 | 117.84 | 124.27 | 118.22 | 121.78 | |||||
| B-O1-H | 115.98 | 112.87 | 116.77 | 114.32 | 117.7 | 117.85 | 123.59 | 116.22 | ||||||
| B-O2-H | 112.65 | 115.67 | 116.59 | 114.16 | 117.37 | 113.82 | 117.77 | 115.81 | ||||||
| Dihedral angle (°) | ||||||||||||||
| C2-C1-B-O1 | 21.3 | 0 | 0 | 0 | -35.61 | 0 | 0 | 0 | -35.05 | |||||
| C2-C1-B-O2 | -159.4 | 180 | -179.98 | 180 | 144.54 | -180 | -180 | 180 | 145.03 | |||||
| C6-C1-B-O1 | -158.2 | 180 | -180 | -180 | 144.33 | -180 | -179.98 | -180 | 144.97 | |||||
| C6-C1-B-O2 | 21.14 | 0 | 0.03 | 0 | -35.53 | 0 | 0.02 | 0 | -34.94 | |||||
Note: anti-syn: a-s, syn-anti: s-a, syn-syn: s-s, anti-anti: a-a.
Table 2: The selected structural parameter of the stable conformers.
When evaluated energetically in possible conformers anti-syn conformer is most stable than other conformers. The energy difference value (ΔE=Ei-Eanti-syn, i=syn-anti, syn-syn and anti-anti, respectively) between conformers increase in the order anti-syn<syn-anti (up to 0.248) <syn-syn (1.465) <anti-anti (3.855 kcal/mol) for B3LYP/6-311++G(d,p) levels of the theory and in the order anti-syn<syn-anti (up to 0.227) <syn-syn (1.0785) <anti-anti (4.577 kcal/mol) for HF/6-311++G(d,p) method. In other words, anti-syn conformer is approximental up to 0.248 kcal/mol more stable than syn-anti up to 1.465 kcal/mol more stable than syn-syn, up to 3.855 kcal/mol more stable than anti-anti and up to 0.227 kcal/mol more stable than syn-anti up to 1.0785 kcal/mol more stable than syn-syn up to 3.577 kcal/mol more stable than anti-anti, respectively. Also, relative energy shows that anti-syn conformer is the most stable, the remaining syn-anti, syn-syn and anti-anti conformers are in range of 0.248-3.855 kcal/mol (HF) and in range of 0.227-3.577 kcal/mol (B3LYP). For molecule, these values are only in range of 1-4 kcal/mol [32]. It shows that the relative energy values obtained in this study are compatible with the values in the literature.
Molecular electrostatic potential and frontier molecular orbitals
Molecular Electrostatic Potential (MEP) describes information about the net electrostatic impact generated at that point by total charge distribution (electron+proton) of around a molecular system. The Molecular Electrostatic Potential (MEP) surface gives information on the reactive sites. The color scheme is as follows, red demonstrates areas of most electro negative EP, blue demonstrates areas of most positive EP and green demonstrates areas of zero potential. The EP increases in the order red<orange<yellow<green<blue. MEP surfaces are plotted over equilibrium geometries of all the conformers at B3LYP/6-311++G(d,p) and HF/6-311++G(d,p) level of theory. From Figure 4 shows that the MEP contours map of conformers. As can be seen Figure 4, in syn-syn conformer has an electrophilic area (red color) around nitrile group (−C≡N) and a nucleophilic area (blue color) around two hydroxyl groups, anti-anti conformer has an electrophilic area around both nitrile group (−C≡N) and two oxygens atoms in boronic acid and other conformers have an electrophilic domain around both the nitrile group (−C≡N) and the oxygen atom attached to the hydrogen atom in the anti-state, while about the oxygen atom attached to the hydrogen atom in the syn-state compose the nucleophilic site.
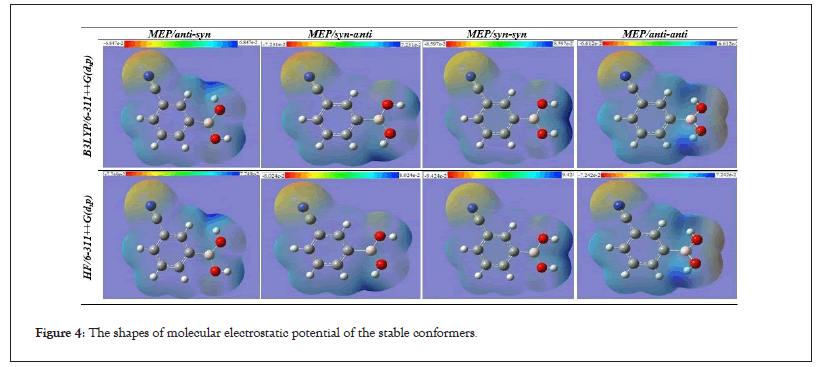
Figure 4: The shapes of molecular electrostatic potential of the stable conformers.
Frontier molecular orbitals and their features like energy are important in the physicists and chemists. The HOMO represents electron donor and LUMO represents electron acceptor. The HOMO and LUMO plots of all the conformers are shown in Figure 5. As seen in the Figure 5, the frontier molecular orbital HOMO of all the conformers calculated in both methods have exhibited similar behavior and the charge density have localized nitrile group and phenyl ring. The frontier molecular orbital LUMO of all the conformers calculated in both methods have not exhibited similar behavior but LUMO calculated at B3LYP/6-311++G(d,p) method have not exhibited similar behavior.
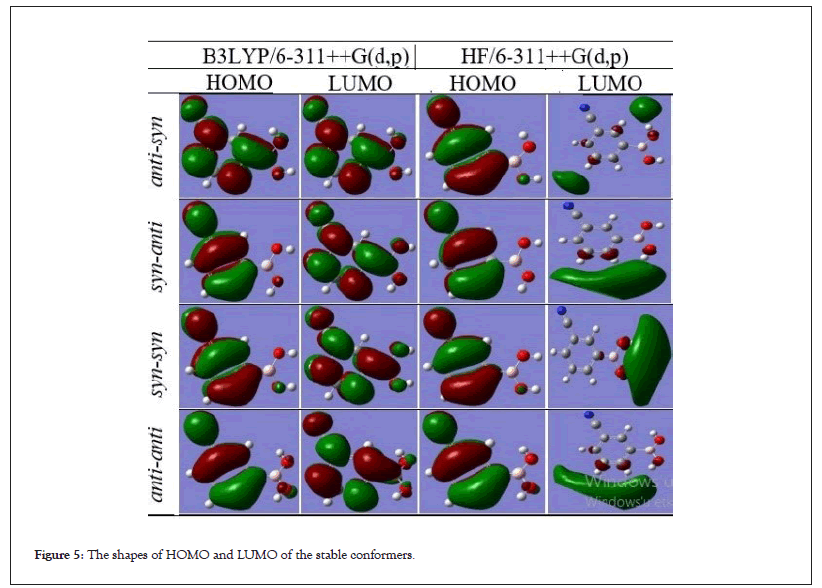
Figure 5: The shapes of HOMO and LUMO of the stable conformers.
Vibrational analysis
3-CyBA molecule consists of 17 atoms, with 45 normal vibrational modes. The stability of the optimized structures of all the conformers has been affirmed by vibration modes calculation, which gave positive values for entire vibration modes. While three conformers, syn-ant, syn-syn and anti-anti, of 3-CyBA molecule belong to C1 symmetry, anti-syn a conformer belongs to Cs symmetry. The obtained theoretically vibrational frequencies are generally higher than the corresponding experimental quantities owing to the combination of electron correlation effects and basis sets deficiencies. So, calculated vibrational frequencies were scaled suitable scale factors. For B3LYP/6-311++G(d,p), scaling factors are used as 0.958 for greater and 0.983 smaller than 1800 cm-1 and for HF/6-311++G(d,p) as 0.906, respectively. The selected wavenumbers data of all the conformer were summarized in Table 3. Additionally, the theoretical IR and Raman spectra are displayed in Figures 6 and 7 respectively. The assignments of vibrational numbers are calculated by employing The VEDA 4f program based on potential energy distribution. OH, CH and CN vibration analyzes of all conformers were performed and characterized. OH, CH and CN vibrations in the functional group region were observed to be compatible with the literature. In particular, when the CN vibration was examined, it was calculated that the CN vibration, which was determined as 2232 cm-1 [40] in the literature, was in the range of 2232-2235 cm-1 in the study. It was determined that the vibrations in the fingerprint region were the same in all four conformations examined and it was observed that they were not affected by the conformation. It was concluded that all the values calculated in the study were consistent with the values in the literature.
| Anti-syn | ||||||
|---|---|---|---|---|---|---|
| Unscaled | Scaled | IR Raman | Raman-Raman | Selected vibration types | ||
| 3888 | 3725 | 43.7 | 44.9 | υO2H(51) υO1H(48) | ||
| 3848 | 3686 | 97.2 | 183.4 | υO2H(48) υO1H(52) | ||
| 3200 | 3066 | 5.7 | 189.9 | υC2H(24) υC4H(01) υC5H(19) υC6H(46) | ||
| 3199 | 3065 | 1.7 | 71.7 | υC4H(56) υC5H(12) υC6H(31) | ||
| 3182 | 3048 | 2.4 | 63.5 | υC2H(60) υC5H(32) | ||
| 3140 | 3008 | 11 | 55 | υC2H(15) υC4H(33) υC5H(36) υC6H(16) | ||
| 2333 | 2235 | 41.2 | 467.5 | υNC7(89) υC7C3(11) | ||
| Syn-anti | ||||||
| 3888 | 3725 | 45 | 50.6 | υO2H(51) υO1H(48) | ||
| 3848 | 3686 | 97 | 173.7 | υO2H(48) υO1H(52) | ||
| 3200 | 3066 | 1.2 | 99.8 | υC2H(24) υC4H(01) υC5H(19) υC6H(46) | ||
| 3199 | 3065 | 3.8 | 110 | υC4H(56) υC5H(12) υC6H(31) | ||
| 3182 | 3048 | 7.3 | 87.6 | υC2H(60) υC5H(32) | ||
| 3140 | 3008 | 20.9 | 86.1 | υC2H(15) υC4H(33) υC5H(36) υC6H(16) | ||
| 2333 | 2235 | 35.2 | 467 | υNC7(89) υC7C3(11) | ||
| Syn-syn | ||||||
| 3871 | 3709 | 110.8 | 365.8 | υO2H(98) | ||
| 3867 | 3705 | 16.6 | 34.6 | υO1H(98) | ||
| 3198 | 3063 | 6.8 | 211.6 | υC4H(14) υC6H(82) | ||
| 3196 | 3062 | 0.8 | 25.2 | υC2H(19) υC4H(39) υC5H(32) υC6H(10) | ||
| 3188 | 3054 | 4.6 | 80.5 | υC2H(72) υC4H(21) | ||
| 3173 | 3040 | 1.9 | 56.5 | υC4H(26) υC5H(66) | ||
| 2331 | 2233 | 44.5 | 471 | υNC7(89) υC7C3(11) | ||
| Anti-anti | ||||||
| 3892 | 3728 | 3.4 | 87.5 | υO2H(98) | ||
| 3891 | 3727 | 111 | 2.4 | υO1H(98) | ||
| 3200 | 3066 | 3.4 | 152.7 | υC4H(38) υC6H(51) | ||
| 3182 | 3049 | 6.4 | 108.5 | υC2H(58) υC5H(29) υC6H(12) | ||
| 3169 | 3036 | 3.2 | 49.1 | υC2H(28) υC4H(27) υC5H(22) υC6H(23) | ||
| 3155 | 3022 | 6.6 | 72.9 | υC2H(10) υC4H(34) υC5H(42) υC6H(13) | ||
| 2334 | 2236 | 33 | 468 | υNC7(89) υC7C3(11) | ||
Note: υ: Upsilon used to represent vibrational energy.
Table 3: The selected structural parameter of the stable conformers.
Figure 6: The shapes of the HOMO-LUMO orbitals of complexes 1-3 and HL at the B3LYP/MIX.
Figure 7: The shapes of the HOMO-LUMO orbitals of complexes 1-3 and HL at the B3LYP/MIX.
The selected wavenumbers data of all the conformer were summarized in Table 3. Additionally, the theoretical IR and Raman spectra are displayed in Figures 6-9. The assignments of vibrational numbers are calculated by employing The VEDA 4f program based on potential energy distribution.
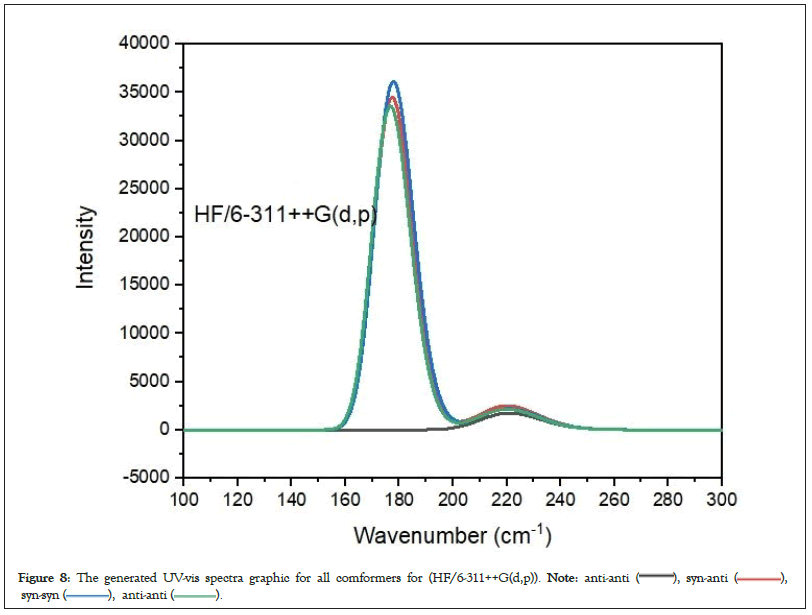
Figure 8: The generated UV-vis spectra graphic for all comformers for (HF/6-311++G(d,p)). 
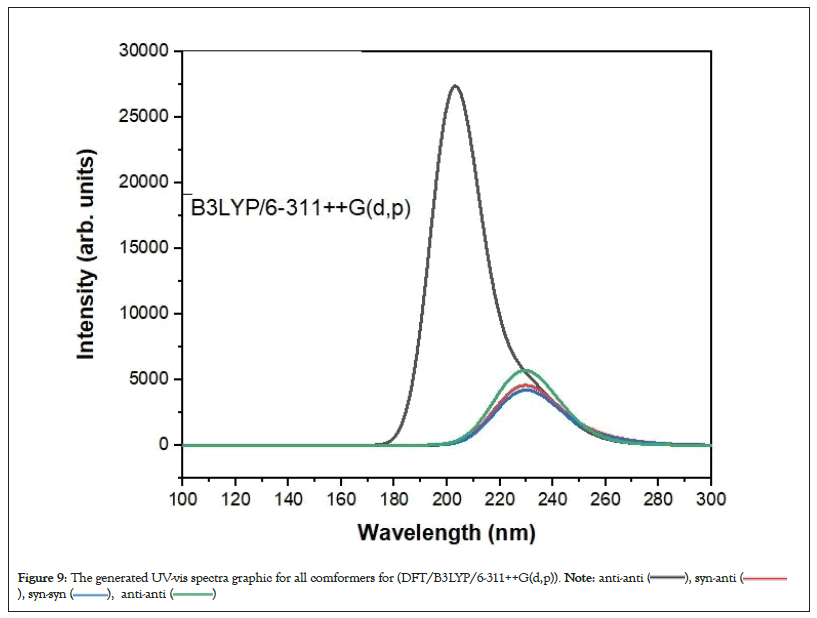
Figure 9: The generated UV-vis spectra graphic for all comformers for (DFT/B3LYP/6-311++G(d,p)). 
UV-vis spectral properties
The UV-vis absorption spectra of the 4 conformer of 3-CyanophenylBoronic Acid (3-CyBA) were investigated (Figures 8 and 9). Calculations were made according to TD-HF and TD-DFT/B3LYP methods using optimized structure.
The 4 conformers of 3-CyBA revealed three absorption bands at the theoretically derived electronic spectra (Figures 8 and 9). Absorption bands in the 300-200 nm range represent π->π* electronic transitions in the benzene ring [41]. As a result, according to these units, All conformers of 3-CyBA have a longer UV-vis absorption wavelength. π-π* transitions are observed in the range of 254.83-202.93 nm originating from the benzene ring in its structure in its 4 conformer. GaussSum 3.0 software was used to determine the stimulation contributions to the UV-visible transitions (Table 4) [42]. For TD-DFT and TD-HF calculations, the primary transition contribution from HOMO-1 to LUMO (56-40%) was calculated as π>π* transitions at 255.11-252.02/221.30-220.89 nm, the primary transition contribution from HOMO to LUMO (68-50%/) was calculated as π>π* transitions at 229.73-229.24/177.81-176.07 nm and the primary transition contribution from HOMO-2 to LUMO (98-91%) was identified as π->π* transitions at 214.85-202.93/370.37 nm for anti-syn, syn-anti, syn-syn and anti-anti conformers. Table 4 displays the predicted excitation energies, absorption wavelengths (λ) and oscillator power (f) of the all conformers.
| λ (nm) | Excitation energy (cm-1) | Oscillator power (f) | Main transition | |
|---|---|---|---|---|
| B3LYP/HF | B3LYP/HF | B3LYP/HF | B3LYP/HF | |
| Anti-syn | 254.07/221.30 | 4.8799/5.6026 | 0.0033/0.0387 | H-1->L (42%), H->L+1 (39%)/H->L+4 (56%) |
| 229.73/218.45 | 5.3971/5.6757 | 0.1084/0.0044 | H->L (68%)/H-1->L+4(50%) | |
| 202.93/176.07 | 6.1097/7.0417 | 0.6720/0.0001 | H-1->L (44%), H->L+1 (50%) | |
| Syn-anti | 254.83/220.89 | 4.8653/5.6129 | 0.0122/0.0376 | H-1->L (39%), H->L+1 (30%) / H->L+3 (40%) |
| 229.24/219.12 | 5.4085/5.6583 | 0.1107/0.0241 | H->L (58%)/H-1->L+3 (40%) | |
| 203.14/177.39 | 6.1035/6.9895 | 0.0001/0.8516 | H-2->L (92%), H->L+7 (48%) | |
| Syn-syn | 255.51/221.21 | 4.8523/5.6049 | 0.0093/0.0398 | H-1->L (36%), H->L+1 (30%)/H->L+6 (51%) |
| 229.66/219.03 | 5.3986/5.6605 | 0.1023/0.0144 | H->L (56%)/H-1->L+6 (48%) | |
| 214.85/177.81 | 5.7707/6.9727 | 0.0001/0.8921 | H->L+2 (98%)/H-1->L+8 (33%) | |
| Anti-anti | 252.02/220.54 | 4.9197/5.6218 | 0.0035/0.0424 | H-1->L (54%), H->L+1 (43%)/H->L+3 (56%) |
| 229.34/217.93 | 5.4062/5.6891 | 0.1396/0.0106 | H->L (81%)/H-1->L+3 (54%) | |
| 208.00/176.72 | 5.9607/7.0158 | 0.0016/0.8291 | H-2->L (91%)/H-1->L+6 (46%) |
Note: H: HOMO AND L: LUMO.
Table 4: The experimental-theoretical UV-vis, oscillator power and the primary transition contribution values with TD-DFT/B3LYP and TD-HF levels of all conformers.
In this work, the Lowest Unoccupied Molecular Orbital (LUMO) and the Highest Occupied Molecular Orbital (HOMO), structural parameters, dipole moments and nonlinear optical properties of all the conformers of 3-CyBA were calculated by both HF/6-311++(d,p) and B3LYP/6-311++(d,p) level of theory. The relative stability of four conformers, anti-syn, syn-anti, syn-syn, anti-anti is 0.00, 0.227, 1.078, 4.577 kcal/mol (HF) and 0.00, 0.248, 1.465, 3.855 kcal/mol (B3LYP), respectively. In both methods, relative energy values of conformers increase in the order: syn-anti<syn-syn<anti-anti. For phenylboronic acid derivative, the values in literature are 1-4 kcal/mol, nearly. The relative energy results show that relative energy values of all the conformers obtained at both methods are in agreement with values in literature. OH, CH and CN vibration analyzes of all conformers were performed and characterized. OH, CH and CN vibrations in the functional group region were observed to be compatible with the literature. It was determined that the vibrations in the fingerprint region were the same in all four conformations examined and it was observed that they were not affected by the conformation. It was concluded that all the values calculated in the study were consistent with the values in the literature.
X-ray crystallography data for the title compound (CCDC 1825335) have been deposited with the Cambridge Crystallographic Data Center.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
Citation: Ugurlu G (2023) The Quantum Mechanical Computations of the Conformational, Structural, Electronic and Spectroscopic Properties of 3-Cyanophenylboronic Acide. J Clin Chem Lab Med. 6:268.
Received: 30-May-2023, Manuscript No. JCCLM-23-24596; Editor assigned: 01-Jun-2023, Pre QC No. JCCLM-23-24596 (PQ); Reviewed: 15-Jun-2023, QC No. JCCLM-23-24596; Revised: 22-Jun-2023, Manuscript No. JCCLM-23-24596 (R); Published: 29-Jun-2023 , DOI: 10.35248/JCCLM.23.6.268
Copyright: © 2023 Ugurlu G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.