Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2017) Volume 8, Issue 2
Four possible pathways of H2S decomposition into hydrogen and elemental sulfur are considered. In the thermal reversible process, H2S dissociation results in the formation of diatomic both hydrogen and sulfur in the singlet state according to the rule of spin conservation:
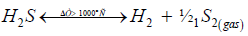 (i)
(i)
On the surface of sulfide catalysts, irreversible H2S splitting proceeds at low temperature through the stage of disulfane, H2S2 formation as a key surface intermediate, followed by its decomposition due to release of hydrogen into the gas phase and recombination of the adsorbed singlet sulfur into cyclooctasulfur:
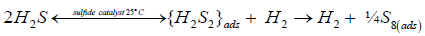 (ii)
(ii)
On metal catalysts, irreversible H2S decomposition occurs at low temperature through the stage of H2S dissociation into the adsorbed atomic surface species resulted in the formation of both diatomic reaction products in the ground electronic state -the singlet hydrogen and the triplet diatomic sulfur:
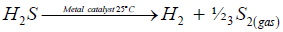 (iii)
(iii)
The reactions (ii) and (iii) are considered to be realized under the principles of biological thermodynamics, in the absence of catalyst both reactions are thermodynamically prohibited in the gas phase. The mechanism of H2S assimilation by sulfur bacteria is suggested to occur in the processes of chemosynthesis resulted in the formation of colorless sulfur globules and activated hydrogen:
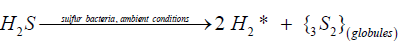 (iv)
(iv)
Similar to the bacterial process, the hydrogen production from H2S is realized with the efficiency of 99.6% on metal catalysts immersed into the liquid which is capable well-dissolving H2S.
Keywords: H2S decomposition; Hydrogen production; Classical and biological thermodynamics; Reaction mechanism; Diatomic sulfur; Triplet and singlet sulfur; Sulfide and metal catalysts; Sulfur bacteria
Hydrogen sulfide is one of the most toxic substances produced as an obligatory and unavoidable by-product in the mining and processing industries in the total annual amount of tens and hundreds of millions of tons. The reserves of hydrogen sulfide in the entrails and water reservoirs of the Earth are estimated by the billions of tons, its contents in the explored deposits of natural gas may be more than 50%. At the same time, it is the "useless" matter, which did not find any commercial application. Therefore, H2S must be removed from waste gases and waters to the level of sanitary norms. Processes of hydrogen sulfide utilization are implemented worldwide by the Claus method, developed in the 19th century; as a result, the final products of its disposal are water and solid sulfur.
H2S, on the other hand, is a simple molecule which has a structure similar to that of water, however, unlike water, the H2S molecule is much less stable and is decomposed into elements even at temperatures as low as 500°C [1]. This means that hydrogen can be released from H2S by an array of techniques including simple heating, irradiation, electrolysis, photolysis etc. [2,3]. Several of these processes have been demonstrated on a significant scale, but none of them has yet achieved commercial success because of high cost of hydrogen produced [4,5]. The goal of this article aims to consider the processes of H2S decomposition from two opposite standpoints. For high-energy, reversible processes, when the system is continuously supplied with energy in the form of heat, the decomposition of hydrogen sulfide is subject to the laws of classical equilibrium thermodynamics for isolated systems, which are permeable neither to heat nor matter. For processes at room temperature, an external source of energy is absent, so the decomposition of hydrogen sulfide is examined from the standpoint of biological thermodynamics, when exchanges of both matter and energy take place in an open system [6].
Thermal dissociation of H2S is a well researched route for the hydrogen production:
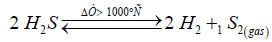 (1)
(1)
Since conversion is very low, only 15% at 1000 °C and 1 atm., it is beneficial to increase the reaction temperature. Rapid quenching the reaction products is important to maximize H2 yields. To shift the reaction equilibrium to the right, various ways of intermediate removing the reaction products have been presented [2,3]. Reversibility of the reaction (1) means that process runs equally well forwards and backwards and is always in a near equilibrium state. In accordance with the rule of spin conservation, diatomic sulfur formed in the reaction (1) is in the metastable singlet state which does not convert into the ground triplet state even at high temperature and in the presence of metal catalysts [7-9]:
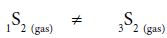 (2)
(2)
As is well known, diatomic sulfur is isoelectronic to oxygen [10,11], therefore the S2 ground state is a triplet X3Σ-g and the first exited state is a singlet a1Δg which is above the ground state by ∼4400 cm-1 (Table 1). However, unlike to singlet oxygen which is spontaneously converted into the ground triplet state [12], the diatomic singlet sulfur prefers to aggregate from the gas phase into the solid S8 structure. Another highenergy processes of H2S decomposition reviewed in [2,3] resulted in the formation of solid sulfur as well.
| State | Te* O2 | Te* S2 |
|---|---|---|
| c 1S-u | 33 057 | 20 203 |
| b 1S+g | 13 195 | 7 981 |
| a 1Dg | 7 923 | 4 395 |
| X 3S-g | 0 | 0 |
Table 1: Comparison of excitation energy (in cm-1) of the three most reliably studied low-lying molecular orbitals of the S2 molecule with a molecule of oxygen O2. Te - energy of electronic term (in cm-1) [11].
Biological thermodynamics is the quantitative study of the energy transductions that occur in and between living organisms, structures, and cells and of the nature and function of the chemical processes underlying these transductions. All biological organisms require energy to survive. No matter what the type of living species, all living organisms must capture, transduce, store, and use energy to live. In application of thermodynamics to biological systems functioning at ambient conditions, the following peculiarities of organization of living systems are accepted [6]:
• biological systems are open to flows of matter and energy;
• processes in living systems are irreversible and far from equilibrium;
• biological systems are heterogeneous, structured, and individual phases may have a small ultimate number of molecules.
All these features distinguish biological systems from the isolated and close to equilibrium systems in the classical thermodynamics. It is well recognized that for an adequate description of the properties of living systems it is necessary to apply the thermodynamics of irreversible processes which deals with the processes in time. A fundamental concept in classical thermodynamics is the notion of equilibrium state. In thermodynamics of irreversible processes an important concept is the notion of a steady state of the system [6]. Unlike thermodynamic equilibrium, the steady state is characterized by a constant flow of substances in and removing the reaction products out of the system, a continuous expenditure of free energy, which maintains the constancy of the concentrations in the system and the constancy of the thermodynamic parameters (including the internal energy and entropy). It is important that an open biological system can exist only due to the inflow of energy from outside and outflow of energy in the environment [6]. Catalytic reactions, which run at ambient conditions (room temperature and normal pressure) and do not obtain energy from the surroundings (solar energy is excluded, we consider only dark processes), seem to be referred to the open systems which are permeable to heat and matter flows [6]. Actually, H2S is a stable molecule at ambient conditions and exists for a long period of time in the absence of action of external forces. However, on passing H2S over metal catalysts at room temperature, two reaction products are observed which are hydrogen and gaseous sulfur [7-9]. Hence, the reaction is likely to occur at the expense of the molecule free energy. The energy stored in H2S can be released through the molecule dissociation on the catalyst surface.
The initial step of any heterogeneous catalytic reaction is an adsorption of substrate molecules on the surface of solid catalyst. The active component of the sulfide hydrodesulfurization (HDS) catalysts consists of a single slab of MoS2 with Ni or Co atoms being located in its edge plane in the center of a square pyramid made of sulfur atoms (Figure 1). The active sites for H2S adsorption are Co(III) or Ni(IV) atoms in the d6 electronic configuration (Figures 2 and 3) [13-15]. The essential element of the active component is hydrogen occluded into the MoS2 matrix (Figures 1 and 3). The occluded hydrogen appeared in the active component during the catalyst sulfiding as a result of its oxidative addition to the Ni (Co) atoms. It localizes in the center of ‘‘empty’’ trigonal prism of a MoS2 matrix made from six sulfur atoms under Ni (Co) atom at the distance of 1.5 Å (Figure 1). The active metal ions are oxidized by the occluded hydrogen to Co(III) and Ni(IV) and acquired d6 electron configuration (Figure 3) [13-15]. Two unoccupied orbitals of the active metal atom, 3dz2 and 3dxz interact effectively with the nσ and nπ orbitals of the H2S molecule (Figure 3). In the absence of occluded hydrogen, H2S molecule is not adsorbed by a Co(II) or Ni(III) atom with d7 electron configuration, because the 3dz2 orbital is occupied. The molecular H2S adsorption on the d6 Co(III) ion is an exothermic process (ΔH298=-12.7 kcal/mol) and occurs spontaneously (ΔG298=-0.8 kcal/mol) [16,17], while H2S adsorption on the d7 Co(II) ion is hardly possible (ΔH298=-2.2 kcal/mol, ΔG298=+6.6 kcal/mol).
Figure 3: Scheme of interaction of nσ and nπ orbitals of a hydrogen sulfide molecule with 3dz2 and 3dxy orbitals of the Co(III) atoms in the d6 electronic configuration [15].
The second step -dissociative chemisorption of two H2S molecules on two adjacent cobalt atoms (Figures 2 and 4), occurs spontaneously (ΔH298=-28.7 kcal/mol, ΔG298=-17.4 kcal/mol) on the d6 Co(III) ions as well, and does not possible for the d7 Co(II) ions (ΔH298>0, ΔG298>0) [16,17]. It is significant that, in the adsorbed state, two H2S molecules interact to one another, as is indicated by their coming together within a short distance of 2.18 Å of each other (Figure 4). As a result, a new sulfur–sulfur chemical bond is formed in the surface intermediate, and this process is accompanied by the release of a hydrogen molecule into the gas phase and by the formation of adsorbed disulfane H2S2 (Figures 4 and 5).
Figure 4: The key surface intermediate {H2S2}ads resulted from H2S dissociation on the surface of sulfide catalysts after H2 molecule being released in the gas phase [16,17].
Atoms of the active component of the sulfide HDS catalysts:
• - sulfur, • - molybdenum, • - cobalt, • - occluded hydrogen, hydride ion H−. Atoms of the adsorbed species:
• sulfur, • hydrogen ion, proton H+.
It is very important, that formation of disulfane, H2S2 from H2S in the gas phase in the absence of catalyst is thermodynamically prohibited:
2 H2S ≠ H2S2+H2 (3)
(ΔrHo298=+13.6 kcal/mol, ΔrSo298=-3.9 cal/mol. K, ΔrGo298=+14.1 kcal/mol, initial parameters for calculations are taken from [18]). Disulfane was also established in the gas phase during the high temperature metal-catalyzed reaction of H2S decomposition [19].
The next step of surface reaction -decomposition of adsorbed disulfane into molecular hydrogen and adsorbed disulfur, is a strongly endothermic process and requires large energy consumption ΔH298=25.5 kcal/mol. There is, however, the possibility of terminating the catalytic cycle, which is the recombination (oligomerization) of molecular sulfur S2 into cyclooctasulfur S8 on the catalyst surface without it’s desorption into the gas phase. This coupled reaction is the highly exothermic process and occurs spontaneously (ΔH298=-78.4 kcal/mol, ΔS298=- 116.0 cal/mol. K, ΔG298=-43.6 kcal/mol [16,17]). Therefore, the overall catalytic reaction:
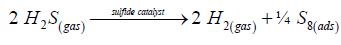 (4)
(4)
runs also spontaneously at room temperature (ΔH298=-23.0 kcal/ mol, ΔS298=-53.2 cal/mol. K, ΔG298=-7.2 kcal/mol [16,17]) (Figure 5). Removal of adsorbed sulfur from the catalyst surface can be realized by immersing solid catalyst into liquid solvent (see next sections).
An unexpected, unpredictable phenomenon was observed when hydrogen sulfide was passed through metal catalysts at room temperature: along with hydrogen, diatomic gaseous sulfur was discovered as a reaction product [7-9,20].
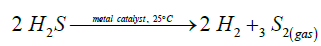 (5)
(5)
This irreversible gasphase reacion occurs on the platinum catalyst at ambient conditions under steady state regime with the effiviency of ∼15%. Let’s consider the reaction (5) in the light of biological thermodynamics. Adsorption of H2S molecule on the surface of any metals is an exothermic process. In the case of Pt(111) [21], the energy of molecular H2S adsorption is Eads=-21.0 kcal/mol. The minimum energy pathway of the adsorbed H2S is then considered for its dissociation in two steps [21]:
H2S(ad) → SH(ad) +H(ad) (6)
SH(ad) → S(ad) +H(ad) (7)
The first dissociation step (6) occurs via the reaction barrier of Ea=1.6 kcal/mol with the reaction energy ΔErxn=-19.6 kcal/mol, while for the second step (7) these values are Ea=0.7 kcal/mol and ΔErxn=- 18.2 kcal/mol. Hence, the total reaction energy of H2S dissociation on Pt(111) into the adsorbed S and H atoms is ΔEdiss.total=-58.8 kcal/mol or ΔEdiss.total=-117.6 kcal/mol for two H2S molecules of the reaction (5). It is very important, that H2S dissociation is a facile process which is kinetically and thermodynamically favorable [21]. On the other hand, from the point of view of thermo-chemistry, the bond dissociation energy Do of HS -H bond in H2S molecule is 92 kcal/mol [18]. In the reaction (5) four HS -H bonds should be cleaved, what is ΣDo=368 kcal/ mol, while two H -H bonds (ΣDo=2 x 104=208 kcal/mol) and one S -S bond Do=102.5 kcal/mol [18] are formed. From this it follows that the total energy of bond dissociation of initial substance is over ΔDo=57.5 kcal/mol than that of reaction products, therefore H2S molecule is stable and does not convert into H2 and S2 at room temperature. However in the adsorbed state the required energy for H2S splitting can be invested from the surface dissociation energy of H2S adsorption. The resulted energy surplus (ΔEdiss.total +ΔDo)=-117.6+57.5=-60.1 kcal/ mol seems to be consumed for desorption of the reaction (5) products into the gas phase. Actually, the adsorption energy of S2 species on Pt(111) is ∼45 kcal/mol [22], while molecular hydrogen desorbs from the surface even at 230 K [23]. Undoubtedly, this rough approximation of energy transduction from H2S dissociation into the surface catalytic reaction should be carefully investigated with both theoretical and experimental methods similar to that made for sulfide catalyst (see above). However, this approach seems to offer new opportunity to understand mechanisms of the low temperature catalytic reactions by means of biological thermodynamics on the atomic scale.
As distinct from solid sulfur, the diatomic sulfur formed in the reaction (5) is well soluble in water, over 5 g of sulfur per liter of H2O [24]. The pH value and refractive index of the colorless sulfur solution are the same as those of the initial water, and the Raman spectrum of the solution exhibits only bands characteristic of water. It is typical of solutions of diatomic gases that do not interact with water (N2, O2, H2, etc.). When the reaction (5) is carried out on metal catalyst immersed into water, white sulfur globules were obtained from the saturated solutions (Figure 6) [24]. The size of the white spherical globules is up to 10 μm, while the smallest globules are practically transparent and colorless. The elemental analysis of the single globule obtained with the energy dispersive spectroscopy evidences in the only element -sulfur [24]. Electron diffraction on the separate sulfur globule indicates a hexagonal structure of the sulfur crystallites with the interplanar spacing of 0.45, 0.29, and 0.15 nm. More than 30 allotropic modifications of solid sulfur are currently known [25,26] with changes in color from red to black, but no white hexagonal sulfur was found in the database. The only single absorption band at 880 cm–1 was observed in the Raman spectra of saturated water solution over precipitate of white globules, while the globules themselves are irreversible transferred into the solid S8 under the laser beam of a Raman spectrometer. Taking into account the hydrophilic character of white globules, we suggest that this substance is obviously composed of S2 condensed phase with sulfur in the ground triplet state.
Surprisingly, the white sulfur globules obtained from H2S on metal catalysts under layer of water bear a great resemblance to colorless globules produced with sulfur bacteria in aqueous medium containing H2S [27-34]. The role of autotrophic (self-feeding) bacteria in the biosphere was first recognized by Winogradsky at the end of 19 century on the example of sulfur bacteria used hydrogen sulfide as an energy source in the chemosynthesis [27]. Bacteria are the simplest living cells which exhibit all the essential features of a living organism. The ability to harness energy from various sources and to channel it into growth and biological work is a fundamental property of these organisms. The bacteria carry out a remarkable variety of energy transductions, conversions of one form of energy to another [6].
There is a wide range of different types of colorless sulfur bacteria with very diverse properties and with equally diverse environmental habitats. The colorless sulfur bacteria are found in marine and freshwater sediments, soils, and waste water treatment systems. They are growing in aqueous media from acid to alkaline pH values, at temperature ranging from +4 to +95°C, in both aerobic and completely anaerobic conditions [30,32-34]. Many of the properties of “biological sulfur” S0 produced with sulfur bacteria do not match the properties of any known chemical allotrope of elemental sulfur [25,26]. S0 forms transparent droplets in diameter of up to 1 μm which are deposited inside or outside of the bacteria cells. S0 is hydrophilic and of white or pale-yellow color. The buoyant density of S0 has been determined as 1.22 g/cm3 [35] in contrast to density of liquid sulfur 1.80 g/cm3 and solid S8 2.07 g/cm3. Therefore, the question was raised of whether S0 is a new form of elemental sulfur or rather sulfur-rich compound [28].
To answer this question, several models of known sulfur compounds were critically evaluated and it was concluded that S0 most likely consists of long-chain polythionates [28]. However, the purposeful searching experiments have shown complete absence of polythionates and a new model was proposed where S0 globules were composed of spherical sulfur droplets, each covered by a monomolecular layer of an amphiphilic compound [36]. Later [32,37] some other models were considered. S0 was also found in the phototrophic organisms under aerobic conditions [38].
It is generally accepted that sulfur bacteria oxidize H2S along a pathway that includes the formation of zero-valent sulfur as a characteristic intermediate [32,34]:
2 H2S+O2 → 2 {S0}+2 H2O (8)
As believed, the energy from this reaction is used to reduce carbon dioxide to create carbohydrates. However, following this reaction (8), hydrogen is irreversibly bound into water and can’t participate in the chemosynthesis. At the same time, it is well recognized that hydrogen is the most important inorganic energy carrier in anaerobic pathways in nature. H2 can be detected in all environments including waters and the atmosphere. Hydrogen does not accumulate but is rapidly recycled where it is produced, and remains at very low concentrations in aquatic environments. H2 is utilized as an energy source by many anaerobic bacteria including sulfate reducers [39]. Therefore, it is not clear how H2 is produced in oxidic waters from H2S if it follows to the reaction (8).
The astonishing resemblance between the hydrophilic globules isolated by us from saturated aqueous solutions upon hydrogen sulfide decomposition (Figure 6) and the globules of biological sulfur produced by colorless sulfur bacteria [27-34,38] suggests that hydrogen sulfide assimilation by these bacteria can be also resulted in the formation of diatomic sulfur in the ground triplet state via the reaction:
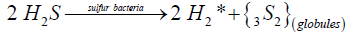 (9)
(9)
where H2* is an activated in situ hydrogen participating in chemosynthesis:
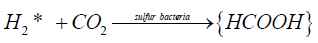 (10)
(10)
Obviously, the reaction (9) takes place as a result of the nucleation of diatomic sulfur as a condensed hydrophilic S2 phase. Since the spherical shape and hydrophilic nature of the globules produced by sulfur bacteria is independent of their nature and habitat [27-34,38], reaction (9) seems to occur both under anaerobic conditions and in the presence of oxygen. Doubtless this hypothesis should be comprehensively verified as well.
Similar to the biological processes of H2S assimilation with sulfur bacteria in aqueous medium, the efficiency of H2S decomposition can be essentially improved (compared to the gas phase process) when solid catalyst is being immersed into a solvent capable of readily dissolving both hydrogen sulfide and the final products of the reaction (5) [20,40].
The use of liquids significantly increases the volumetric and surface concentration of hydrogen sulfide compared with the gas phase. This, in turn, leads to a substantial increase in the overall rate of chemical transformation due to the increasing coverage of the catalyst surface by adsorbed molecules of hydrogen sulfide. Moreover, the reaction product -diatomic sulfur, accumulates in the solvent thereby freeing the catalyst surface for adsorption of new molecules of hydrogen sulfide.
As is well known, purification of any wastes from hydrogen sulfide begins with its extraction from gas streams in absorbers with liquid solvent to the required sanitary standards. Concentrated solutions of the absorbers are thereupon directed to the regeneration stage where H2S is recovered by solvent boiling and is utilized mainly with the Claus method or is used to obtain sulfuric acid. Instead of this high temperature procedure of H2S utilization, the concentrated with H2S solutions could be passed through the metal catalyst at ambient conditions to provide the reaction (5) and to get hydrogen as the target product, while sulfur is accumulated in solution.
Indeed, the aqueous monoethanolamine (MEA) solution, a wellknown and widespread hydrogen sulfide absorber, is turned out one of the most suitable solvent for the reaction (5) realization. Decomposition of H2S occurs at room temperature with hydrogen evolution to the gas phase (Figure 7) and sulfur accumulation in solution with efficiency towards H2S utilization of 97.9% (Table 2) [40]. Efficiency of H2S decomposition can be improved to 99.6% in the periodical regime (Table 3) [20], when, after solution saturation with H2S, the reaction is allowed to be completed in a closed system. No doubt, the possibilities of this process to achieve close to 100% efficiency of H2S decomposition is practically unlimited taking into account a wide range of choice of catalysts, solvents, regimes, etc.
Figure 7: Evolution of hydrogen in the process of H2S decomposition at room temperature on the stainless steel chip immersed into the aqueous 5% MEA solution [40].
| Solution | 5 % MEA | Na2CO3 [Na] = 0.84 % |
|
|---|---|---|---|
| Volume, ml | 200 | 100 | |
| Fed H2S , mmol | 110.6 | 53.4 | |
| H2S decomposed, mmol (g) | 108.3 (3.69) | 42.5 (1.33) | |
| H2S conversion, % | 97.9 | 79.6 | |
| Sulfur content in solution*, | % mass | 1.62 | 1.30 |
| g | 3.24 | 1.30 | |
* - X-ray fluorescent analysis data after removal of non reacted H2S from solution with Ar flow
Table 2: H2S decomposition over stainless steel chips placed into aqueous solutions. Catalyst mass is 5 g. Reaction temperature is ambient, Ar flow is 10 ml/ min, H2S flow is 3 ml/min.
| Adsorption No | Time on stream, min | H2S, mmol | Conversion, % | |
|---|---|---|---|---|
| Fed | Non-reacted | |||
| 1 | 130 | 10.45 | traces | |
| 2 | 111 | 8.91 | traces | |
| 3 | 120 | 9.64 | traces | |
| 4 | 270 | 21.7 | traces | |
| S | 631 | 50.7 | 0.2 | 99.6 |
Table 3: The periodical regime of hydrogen sulfide decomposition at room temperature over stainless steel chips placed in aqueous 5% MEA solution. Argon flow rate, 6 ml/min; H2S flow rate, 1.8 ml/min; catalyst weight, 2 g; solution volume, 150 ml [20]. After saturation with H2S, the reactor inlet and outlet were closed and overnight later the H2S feeding starts again.
Analysis of technologies of H2S disposal has shown that the method proposed can be adapted to the existing industrial technologies of H2S utilization. The implementation of this method on an industrial scale actually means the gradual replacement of the metal- and energyintensive Claus processes for a new mode of operation at ambient conditions to produce hydrogen -a recognized energy resource of the future and a valuable chemical reagent. As liquid absorbers, it is proposed to use the known commercially available solvents of hydrogen sulfide with well proven technology of their operation.
“Useless” and hazardous hydrogen sulfide, which in nature is inexhaustible and renewable, plays a very important role in chemosynthesis as an energy and hydrogen source. Due to the sulfur bacteria, H2S is easily refined into the vitally important substances under ambient conditions in the natural springs, so it is not accumulated in the atmosphere. However it creates a lot of problems in the refinery of petroleum, natural gas and coal, requiring the energy- and metalintensive processes of H2S utilization. Therefore, the catalytic reaction of low-temperature decomposition of H2S into hydrogen and sulfur seems to offer new opportunity to use the biologically-similar process at the industrial scale. The stumbling block of our researches was to explain experimental data in the framework of classical thermodynamics, because it is hardly possible to understand formation of hydrogen and gaseous diatomic sulfur in the ground triplet state from H2S at room temperature. In terms of biological thermodynamics, the role of solid catalysts becomes clear as an energy transducer from the exothermic processes of molecule adsorption to the surface chemical reactions, which are impossible to be done in the gas phase. Therefore, there exist two regions of the reaction of H2S decomposition (Figure 8). At high temperature this reaction follows to the laws of the classical equilibrium thermodynamics and results in formation of both diatomic reaction products in the singlet state. At the ambient conditions, this reaction occurs either on the surface of solid catalysts or in the bacteria cells. However, the final reaction products are different depending on the catalyst and the reaction conditions. Of the special interest is the diatomic sulfur in the ground triplet state X3Σ-g, which can be obtained in the gas phase only (?) on the surface of metal catalysts via H2S decomposition [7-9,20]. The standard thermodynamical reference data [18] are concerned with the metastable single state a 1Δg of this molecule (Table 1). Following the analogy with oxygen, the standard enthalpy of the 3S2 molecule formation should be equal to zero as well. However, the generally accepted thermodynamical reference point is the orthorhombic solid sulfur. Therefore, to solve this conflicting problem, it should be necessary to compare the full energy of the S8 molecule with that of the diatomic triplet sulfur. We made attempts to calculate full energy of cyclooctasulfur by the available computational methods, but unsuccessfully. So, the problem is open for discussion.