Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2016) Volume 7, Issue 3
Diabetes mellitus (DM) is a chronic disease characterized by hyperglycemia and its prevalence is rising globally. Prolonged exposure to uncontrolled chronic hyperglycemia can lead to various complications in the eye including cataract. Cataract is characterized by cloudiness or opacification of the eye lens which is the leading cause of sight loss and visual disability. It has been reported that diabetes mellitus is a major problem in the management of blindness and cataract surgery.
Keywords: Diabetic cataract; Hyperglycemia; Oxidative stress
In recent years, various studies suggested that oxidative stress may play a role in the pathogenesis of both type 1 and type 2 DM and its impact on lens transparency [1-5]. Oxidative stress results mainly by an increased production of free radical and a sharp reduction of antioxidant defense [6]. Antioxidants, that inhibit the destructive effects of oxidants, involve both non-enzymatic, such as ferritin, ascorbate, glutathione and vitamins A & E, and enzymatic strategies such as catalase (CAT), glucose-6-phosphate dehydrogenase (G6PD), glutathione peroxidase (GSH-Px), glutathione-S-transferase and superoxide dismutase (SOD) [7,8].
Various studies reported that the persistent hyperglycemia in both types of diabetic patients produces excess ROS leading to increased oxidative protein damage and lipid peroxidation, which would be related to the pathogenesis of diabetic’s complications including cataract [9-11]. Furthermore, a number of studies have evaluated the oxidant markers in diabetes and its complications but with inconsistent results. While some studies reported increased MDA, a marker of lipid peroxidation and lowered activities of SOD, CAT and GSH-Px in patients of DM [10-13], others have reported no change in indices of oxidative stress [14-16].
Another mechanism involved in sugar cataract formation is the metabolic imbalance of glucose through polyol pathway in diabetic patients. The polyol pathway involves two enzymes: aldose reductase AR; EC 1.1.1.21) and sorbitol dehydrogenase (SDH; EC 1.1.1.14). AR is a member of aldo-ketoreductase family and the first and the key enzyme of the polyol pathway. It reduces glucose to sorbitol using nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor. Sorbitol is then metabolized to fructose by SDH using NAD+ as a cofactor [17].
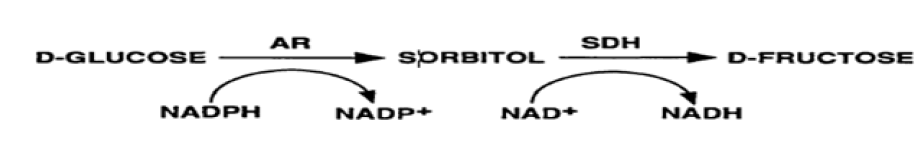
Under hyperglycemic conditions in diabetic patients, there is an increased flux of glucose through the polyol pathway which is reduced to sorbitol catalyzed by AR. The accumulation of sorbitol in the lens, has been linked to the development of diabetic cataract [18]. Ng et al. [19] found the reduction of SDH in hyperglycemic patients, but the results obtained by Omotosho et al. [20] observed that the SDH is elevated in DM but not in diabetic cataract patients.
Several studies have investigated the role of oxidative stress [13,21,22], and the polyol pathway in the pathology of diabetic retinopathy and diabetic cataract [23-25], but, the prevalence of these two pathways in either type of diabetes mellitus was not studied clearly.
Therefore, the present study would investigate the prevalence of antioxidant status and polyol pathway in either type 1 or type 2 or in both and in the diabetic cataract. In addition, the present study also would investigate the relationship between two pathways in the ability of occurrence of diabetic cataract.
Chemicals
Kits required for determination of different parameters under study were purchased from Sigma/Aldrich Chemical Company, St. Louis, MO, USA.
Subjects
The present study included 170 subjects who are divided into 4 groups, group 1 represents type I diabetic patients (n=50) without cataract, group 2 represents type II diabetic patients (n=50) without cataract, group III represents diabetic cataract patients (n=20), and group IV represents the age and gender matched normal control subjects (n=50). The diabetic patients with and without cataract attending the Endocrine and Diabetes out-patient clinic Unit at King Fahd Hospital, Hofuf, Al-Ahsaa, Saudi Arabia referred to the Ophthalmology Clinic to evaluate the diabetic eye complications. The age of both patients and control subjects were between 25-50 years and the period of the diabetes mellitus was 5 years or more. Subjects >50 years old, or who had a prior history of uveitis, ocular trauma or previous eye surgery for other cause than cataract, such as vitrectomy, were not included.
Anthropometry
All patients answered a standard questionnaire and underwent physical examination. Weight was measured using commercial scale ‘‘Seca Germany’’ with an accuracy of ± 100 g. Subjects were weighed in light outdoor clothes without shoes and height was recorded. Body mass index (BMI) was calculated as ‘‘Body weight (kg)/height in meters2”. Sitting blood pressure was measured twice on the right arm to the nearest 2 mmHg after a 10 min rest using a standard mercury sphygmomanometer (phases I and V of Korotkoff’s sounds).
Eye examination
Eye examination included a visual acuity test (log MAR notation), refraction, tonometry and biomicroscopy of the anterior and posterior segments. Cataract was diagnosed based on the Lens Opacities Classification System II (LOCSII) criteria [26] and any grade of lens opacity was classified as the patient having cataract.
Study protocol was approved from authorities at King Faisal University. Eligible subjects signed a written consent.
Blood sample
Fasting blood samples were freshly withdrawn from all subjects under investigation on heparin after an overnight fasting inpatient King Fahd hospital. These blood samples were immediately transferred from the King Fahd Hospital and Medical Center at King Faisal University to our laboratory at the College of Medicine, King Faisal University in an ice box. Each sample was centrifuged at 4000 rpm and separate plasma from RBCs. The plasma was kept at –80°C until used. The RBC was taken and lysed with ice-cold water and the clear lysate obtained after spinning down the cell debris at 8500 g for 10 min at 4°C was used for the assays
Since human red blood cells (RBC) have the metabolic capacity for glucose sorbitol-fructose conversion [27], the present study would determine the most biochemical parameters related to cataract formation in red blood cells.
Laboratory methods
Estimation of fasting blood glucose (FBG): Blood glucose concentration was estimated spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) through application of method described by Freund et al. [28] by using enzymatic test kit (glucose oxidase). The results were expressed as mg/dL.
Estimation of hemoglobin (Hb %): Hb was estimated spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) according to the method of Baure [29]. The values are expressed as g/dL.
Determination of erythrocytes oxidative stress markers
Determination of malondialdehyde level: Malondialdehyde (MDA) concentrations, the end product of lipid peroxidation of erythrocytes were measured spectrophotometrically at 532 nm as the product of the reaction with thiobarbituric acid (TBA) using the method of Dahle et al. [30]. Results were expressed in nmol/g Hb.
Determination of superoxide dismutase (SOD) activity (SOD; EC 1.15.1.1): Halliwell and Gutteridge method [31] was used to estimate the total SOD activity spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) in hemolysate. The results were expressed as U/g Hb.
Determination of glutathione peroxidase (GSH-Px; EC 1.11.1.9): The erythrocyte activity of GSH-Px was estimated spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) by using the method described by Paglia and Valantine [32]. The results were expressed as mU/g Hb.
Determination of glutathione reductase (GSSG-R; ECEC 1.6.4.2): Erythrocyte GSSG-R activity was determined spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) by using the method described by Worthington and Rosemeye [33]. The results were expressed as mU/g Hb
Determination of catalase activity (CAT; EC 1.11.1.6): CAT activity was measured spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) using a standard CAT assay through following the decomposition rate of H2O2 at 240 nm according to the method of Aebi [34]. The results were expressed as U/g Hb.
Determination of reduced glutathione (GSH): GSH was assayed by reaction with dithionitrobenzenoic acid (DTNB) as described by Anderson [35]. The product was quantified spectrophotometically at 416 nm. The results of GSH were expressed as nmol/g.
Polyol pathway markers
Determination of blood aldose reductase (AR) activity: The hemolysate AR activity was measured spectrophotometrically according to the method described by Suryanarayana et al. [36], using Boeco S-20 Spectrophotometer, Hamburg, Germany. One unit was defined as μmol NADPH/g Hb
Determination of blood Sorbitol dehydrogenase (SDH) activity: The hemolysate SDH activity was measured spectrophotometrically by applying the method of Vaca et al. [37], using Boeco S-20 Spectrophotometer, Hamburg, Germany. One unit was defined as U/g Hb.
Statistical analysis
Data were analyzed with the SPSS 16.0.7 (SPSS, Chicago, IL, USA) for Microsoft Windows XP (Redmond, WA, USA) statistical software package. Group comparison was performed by using a one-way analysis of variance (ANOVA). Values are expressed as mean ± standard deviation, and p<0.05 was considered statistically significant.
Basic characteristics
Table 1 displays the basic characteristics of both diabetic patients, diabetic-cataract patients and their controls. Age and gender distribution and body mass index showed no significant difference between the various groups. There is a significant variation in glucose levels between the patients and control subjects.
| Characteristics | Subjects | |||
|---|---|---|---|---|
| Type-I (N= 50) |
Type-II (N= 50) |
Diabetic Cataract (N= 20) |
Control (N= 50) |
|
| - Gender: | ||||
| Males (%) | 37 (74) | 34 (68) | 15 (75) | 36 (72) |
| Females (%) | 13 (26) | 16 (32) | 5 (25) | 14 (28) |
| - Age in years (mean ± SD) |
25.9 ± 5.9 | 26.3 ± 7.7 | 26.1 ± 6.2 | 25.6 ± 6.2 |
| -Diabetes duration (years) (mean ± SD) |
14.6 ± 4.2 | 15.1± 4.8 | 9.9 ± 3.6 | None |
| - Systolic blood pressure (mmHg) (mean ± SD) |
120.3 ± 21.5 | 121.6 ± 22.4 | 122.6 ± 23.2 | 119.6 ±19.6 |
| -Diastolic blood pressure (mmHg) (mean ± SD) |
78.3 ± 10.3 | 79.4 ± 11.6 | 22.1±5.31 | 21.9±4.94 |
| -Body mass index! (mean ± SD) | 78.9± 10.9 | 77.6±9.9 | 22.4 ±5.41 | 22.3±5.24 |
| -Insulin dose U/kg/day) (mean ± SD) |
0.73 ± 0.28 | 12.7 ± 4.0 | 0.95± 0.31 | None |
| -Hemoglobin (gm %) (mean ± SD) |
12.5 ± 4.1 | 12.4 ± 3.9 | 12.8 ± 3.4 | |
| -Fasting blood glucose (mg/dl) (mean ± SD) |
171.7 ± 10.9a | 236 ± 16.5a,b | 89.3 ± 8.7 | 245.9 ± 15.7a,b |
| SD=Standard Deviation ! Body mass index=weight in Kg/height in meter2. aStatistically significant of diabetic and diabetic cataract patients versus control subjects bStatistically significant of type-I and diabetic cataract patients versus type-II patients |
||||
Table 1: Basic characteristics of the study subjects.
Table 2 demonstrates the erythrocytes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px)), and glutathione reductase (GSSG-R) activities in fasting blood samples from type-I, type-II, diabetic cataract and their controls. The activities of erythrocytes SOD, CAT, GSH-Px and GSSG-R were significantly decreased in all patients compared to their control. The activities of these enzymes were significantly decreased in both type-I and diabetic cataract patients compared to type-II patients.
| Subjects | SOD U/g Hb | CAT (U/g Hb) | GSH-Px (U/g Hb) | GSSG-R (U/g Hb) |
|---|---|---|---|---|
| Controls (no.) | (50) | (50) | (50) | (50) |
| Mean ± SD | 20.8 ± 5.1 | 43.9 ± 10.4 | 41.4 ±12.6 | 52.4 ±11.6 |
| Type-I DM (no.) | (50) | (50) | (50) | (50) |
| Mean ± SD | 9.9 ± 2.6 | 20.3 ± 6.1 | 29.6 ± 7.4 | 28.1 ± 7.9 |
| P | 0.0001a,b | 0.0001a,b | 0.0001a,b | 0.0001a,b |
| Type-II DM (no.) | (50) | (50) | (50) | (50) |
| Mean ± SD | 14.7 ± 2.6 | 26.8 ± 3.8 | 36.3 ±9.62 | 33.9 ±9.4 |
| P | 0.0001a | 0.0001a | 0.0001a | 0.0001a |
| Diabetic-cataract (no.) | (20) | (20) | (20) | (20) |
| Mean ± SD | 10.2 ± 3.8 | 18.9 ± 4.9 | 27.3 ± 8.9 | 26.6 ± 6.6 |
| P | 0.0001a,b | 0.0001a | 0.0001a,b | 0.0001a,b |
| SD=Standard Deviation. aStatistically significant of diabetic and diabetic cataract patients versus control subjects. bStatistically significant of type-I and diabetic cataract patients versus type-II patients | ||||
Table 2: Erythrocytes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and glutathione reductase (GSSG-R) activities among type-I, type-II diabetic patients without cataract, diabetic cataract and control subjects.
Table 3 demonstrates the erythrocytes activities of aldose reductase (AR) and sorbitol dehydrogenase (SDH) in diabetic patients without and with cataract. The activity of erythrocytes AR was significantly elevated in hemolysate of types 1 & 2 diabetic patients without cataract and diabetic cataract compared to their controls. The AR activity was significantly increased in type 1 diabetic patients without cataract and diabetic cataract patients compared to that of type 2 diabetic patients without cataract. The erythrocyte activity of SDH was significantly increased in all patients groups compared to their control. The hemolysate SDH activity of type 1 diabetic patients without cataract and of diabetic cataract patients was significantly elevated compared to that of type 2 diabetic patients without cataract.
| Subjects | Aldose reductase (µmol NADPH/g Hb) |
Sorbitol dehydrogenase (U/g Hb) |
|---|---|---|
| Controls (no.) | -50 | -50 |
| Mean ± SD | 18.3 ± 7.2 | 17.6 ± 6.6 |
| Type-I DM (no.) | -50 | -50 |
| Mean ± SD | 36.5 ± 11.6 | 20.2 ± 7.8 |
| P | 0.001a,b | 0.001a,b |
| Type-II DM (no.) | -50 | -50 |
| Mean ± SD | 24.9 ± 9.7 | 18.9 ± 8.4 |
| P | 0.001a | 0.05a |
| Diabetic-cataract (no.) | -20 | -20 |
| Mean ± SD | 40.3 ±14.9 | 22.9 ± 9.7 |
| P | 0.001a,b | 0.0001a,b |
| SD=Standard Deviation.The results are represented by Mean ± SD aStatistically significant of diabetic and diabetic cataract patients versus control subjects bStatistically significant of type-I and diabetic cataract patients versus type-II patients |
||
Table 3: Erythrocyte aldose reductase and sorbitol dehydrogenase activities in two types of diabetic patients without cataract, diabetic cataract patients and the control subjects.
The concentrations of MDA and GSH are shown in Figures 1A and 1B respectively. MDA concentration was significantly increased (Figure 1A), while GSH level was significantly reduced (Figure 1B) in all patients’ groups compared to the corresponding values of control group. In addition, the MDA level was significantly elevated, while GSH level was significantly reduced in type I diabetic patients without cataract and diabetic cataract compared to the values of type II diabetic patients (P<0.001).
Figure 1A: Erythrocytes levels of malondialdehyde (MDA nmol/g Hb) in control, type-I DM without cataract, type-II DM without cataract and diabetic cataract. Results are expressed as mean ± SD. aStatistically significant of diabetic and diabetic cataract patients versus control subjects. bStatistically significant of type-I and diabetic cataract patients versus type-II patients.
Figure 1B: Erythrocytes levels of glutathione (GSH mg/g Hb) in control, type-I DM without cataract, type-II DM without cataract and diabetic cataract. Results are expressed as mean ± SD. aStatistically significant of diabetic and diabetic cataract patients versus control subjects bStatistically significant of type-I and diabetic cataract patients versus type-II patients
Many biochemical pathways have been suggested to study the pathogenesis of diabetic cataract in patients with hyperglycemia [1]. Among these, the oxidative stress and the polyol pathway which have been extensively studied.
Several studies have found that hyperglycemia is associated with enhanced production of ROS leading to oxidative stress in diabetic patients [38-41]. The present data is consistent with these studies and provides further evidence of increased production of ROS with depletion of antioxidants and increased lipid peroxidation (MDA levels) in both types of diabetic patients without cataract as well as diabetic cataract. However, no data are available on the prevalence of oxidant status and polyol pathway in either type of DM.
The present study showed that the activities of SOD, CAT, GSH-Px and GSSG-R were significantly decreased (Table 2), while MDA level was significantly increased (Figure 1A) in all diabetic patients compared to the results of normal subjects. These findings were supported previously in which CAT and SOD activities were decreased in blood of diabetic animals [10,42-44]. The reduction of antioxidant enzymes in the present study may be due to either the inactivation or inhibition of these enzymes by the increased production of ROS during diabetes [45-47], or the excess production of malondialdehyde (MDA) which have additional toxic effects on proteins including enzymes. MDA can modify the amino acid side chain of these enzymes results in a partial or complete loss of functions of their functions leading to decrease in their activities [48]. In addition, the elevation of MDA (Figure 1A) in the present study may be also attributed the excess production of ROS in hyperglycemia through autoxidation and nonenzymatic protein glycation which promote erythrocytes lipid peroxidation leading to excess production of MDA [49].
In hyperglycemic patients of the present study, the significant reduction in erythrocytes SOD activity may be attributed to the progressive glycation of SOD [50]. In addition, the reduction of erythrocytes CAT and GSH-Px activities in the present study may be attributed to the reduction of SOD activity (Table 2). This finding is supported by the studies of Kono et al. [51], and Blum et al. [52], which found that the decrease in SOD activity may lead to increase level of superoxide radicals (O2.-) which will cause the inactivation of CAT and GSH-Px. The present data is also confirmed by the results obtained by Du et al. [53], who found that the elevated glucose concentration enhances the production of superoxide anion (O2.-) by retina and retinal cells.
Furthermore, in hyperglycemia, the increased AR activity may impair antioxidative defense enzymes, including SOD, GSH-Px, CAT, and GSSG-R in diabetic patients. The mechanism of the impaired effect of AR on these antioxidative enzymes may be attributed to that AR may affect gene expression of such antioxidative enzymes in diabetic subjects [54,55].
Glutathione (GSH) has many important functions, such as it acts as a direct cellular antioxidant for removing oxidative species, as an essential co-substrate for GSH-Px, and as a cofactor for many enzymes [56]. The present data observed that GSH concentration was decreased in erythrocytes of both types of DM groups and diabetic cataract subjects compared to the non-diabetic subjects (Figure 1B). The depletion of GSH may be due to the excess production of ROS in these diabetic patients which consume GSH [57]. GSH is considered as a cofactor for GSH-Px, thus the reduction in GSH level will cause a reduction in GSH-Px activity which is observed in Table 2 of the present study [58].
Results of the present study indicated that the oxidative stress generated by antioxidants depletion and MDA accumulation may be involved in the development of cataract associated with diabetes. This finding is supported by evidence obtained from clinical and animal studies suggested an association between oxidative stress and development of diabetic cataract [59,60]. Furthermore, the involvement of oxidative insults in the pathogenesis of diabetic cataract was also indicated by the delay in the progression of cataract development in mice (of loss antioxidants) treated with antioxidants butylated hydroxytoluene [61], vitamin C [62] and vitamin E [63].
The present results showed that the AR activity, which is considered as key enzyme of polyol pathway, was highly elevated in diabetic subjects (Table 3). AR activity is increased with increasing glucose levels as in hyperglycemia, because AR has a very high Km for glucose. Thus, the rate of reduction of glucose to sorbitol increases with increasing glucose levels in tissues that do not require insulin for glucose uptake like lens due to the AR activation [37,64].
On the other hand, SDH activity was significantly increased in both type I DM and diabetic cataract patients but slightly increased in type II DM, compared to its value in normal individuals (Table 3). The increased activity of erythrocytes SDH in all types of diabetic patients may be attributed to the aldose reductase catalyzed accumulation of erythrocytes sorbitol [65].
NADPH is the cofactor for both AR & GSSG-R. Thus, the competition between AR and GSSG-R for the cofactor NADPH in the hyperglycemic state may cause GSH depletion. The increased AR activity in polyol pathway in DM consumes NADPH, results in impaired activity of GSSG-R leading to depletion of GSH. The depletion of GSH impairs the activity of antioxidant enzymes, e.g. GSH-Px as well as that of chain breaking aqueous and lipid phase antioxidants. These results reflect the contribution of AR in increased oxidative stress resultant oxidative damage which can then contribute to pathogenesis of DM [66-68]. These findings are consistent with a number of studies supported the role of activated AR in increasing of oxidative stress in diabetic complications. The studies of Chung et al. [67] and Obrosova [69] reported that the activation of AR enhanced lipid peroxidation in tissue sites for diabetic complications. In addition, the administration of aldose reductase inhibitors (ARIs) in diabetic cataract rats has restored the GSH, lipid peroxidation (LPO) and the activities of antioxidative enzymes to near normal levels [54,70,71].
There is accumulating evidence showing the contribution of sorbitol dehydrogenase (SDH), the second enzyme in the polyol pathway that converts sorbitol to fructose, in the development of oxidative stress in diabetic patients. This finding was supported by the study of Tilton et al. [72], which showed that administration of SDH inhibitors (SDHIs) into diabetic rats attenuated the diabetes-induced increase in cytosolic NADH/NAD+ ratio and oxidative stress in diabetic retina. The mechanism through which SDH contributes in development of oxidative stress may be related to that the activation of SDH produces excess of NADH which, in turn, leads to more glucose being channeled through the polyol pathway and enhanced NADH oxidase which generates excess of ROS and thus oxidative stress [69,73].
The oxidative stress and the polyol pathway were significantly higher in insulin dependent diabetes mellitus (IDDM) patients and diabetic cataract than controls and non-insulin dependent diabetes mellitus (NIDDM) patients (Tables 2 and 3 and Figure 1A and 1B) which may be attributed to the excessive ROS generation in IDDM patients. The present findings were supported by previous studies which reported significant decrease in glutathione peroxidase, catalase and glutathione, and significant increase of MDA concentration [74] and AR activity [75] in type 1 diabetic patients respectively.
The present observations showed the contribution of oxidative stress and polyol pathway in the development of diabetic cataract among diabetic patients, particularly type 1 DM. Furthermore, polyol pathway contributes to redox imbalance in diabetic tissues in oxidative stress via depletion of GSH in diabetic patients particularly type 1 DM.