Journal of Cell Science & Therapy
Open Access
ISSN: 2157-7013
ISSN: 2157-7013
Mini Review - (2022)Volume 13, Issue 2
Bile acids are critical endocrine elements of the signalling pathway regulating important pathophysiological processes in the diverse liver and biliary diseases. This mini-review summarizes the interplay between bile acids, bile acids activated receptors, and host-microbiome in hepatic and biliary disorders, such as non-alcoholic fatty liver disease, cholestatic liver disease, and HBV-related liver disease, and discusses potential BAs-based therapeutic strategies for these diseases.
Bile acids; Non-alcoholic fatty liver disease; Primary biliary cholangitis; Primary sclerosing cholangitis; HBV-related liver disease
Bile Acids (BAs) are small molecule metabolites essential for maintaining cholesterol homeostasis, lipid and energy metabolism, and gut microbiota homeostasis in the liver and intestine. Abnormal BAs metabolism causes inflammatory and fibrogenic cellular dysfunction, which eventually drives diverse liver and biliary diseases, such as non-alcoholic fatty liver disease, cholestatic liver disease, and HBV-related liver disease [1-3]. BAs' synthesis, metabolism, and distribution are mediated via the intricate relationship between BAs, BAs-activated receptors, and the intestinal microbiome. Serving as the main components of bile, BAs are converted from cholesterol through the Cytochrome 7α Hydroxylase (CYP7A1)-mediated classic pathway and mitochondrial Cytochrome 27α Hydroxylase (CYP27A1)-mediated alternative pathway in the liver. Both enzymes are transcriptionally regulated by BAs activated nuclear receptor FXR [4]. The primary BAs, such as Cholic Acid (CA), Chenodeoxycholic Acid (CDCA), and their conjugated forms, are excreted into the small intestine to emulsify and assimilate dietary fat and fat-soluble vitamins. The conjugated CA and CDCA undergo deconjugation and dehydroxylation by resident bacteria in the distal small intestine and colon to generate secondary BAs, such as Deoxycholic Acid (DCA) and Lithocholic Acid (LCA), which were reabsorbed passively with the enterohepatic circulation and formed a part of the total BA pool. The growing evidence that BAs regulated signalling control liver and biliary homeostasis in physiology and pathological conditions has intrigued strong enthusiasm to explore the therapeutic potential of BAs in hepatic and biliary diseases [5,6].
Non-Alcoholic Fatty Liver Disease (NAFLD) is a common chronic liver disease characterized as simple hepatic steatosis to progressive Non-Alcoholic Steatohepatitis (NASH) with or without fibrosis and cirrhosis. NAFLD/NASH patients presented enhanced BAs in their circulation, liver tissue, and feces, likely due to increased primary bile acid synthesis or induced secondary bile acid converted from the intestinal microbiota [4].
As potent endogenous activators of FXR, the percentage of CDCA was reduced along with elevated fibrosis stage in NASH patients, while the proportion of FXR antagonistic DCA was increased [4,7]. DCA initiated DNA damage and hepatocellular ballooning via inducing reactive oxygen species, contributing to liver injury and inflammation in NASH [7]. UDCA has been implicated as a protective factor to mitigate bile acid-induced hepatotoxicity. Increased fecal excretion of UDCA is significantly correlated with fibrosis severity, especially in non-obese subjects with NAFLD [3]. Abnormal changes in the composition of BAs undermined FXR-mediated and FGFR4-mediated signaling in the liver and the intestine of NASH models, resulting in dysregulation of key regulatory genes involved in BA metabolism, including CYP7A1, Na+-Taurocholate Cotransporting Polypeptide (NTCP), Organic Solute Transporter alpha-beta (OSTα/β) and Bile Salt Export Pump (BSEP), which further affects the uptake, synthesis, and basolateral exportation of BAs [8].
These abnormal BAs profiles can be exploited as biomarkers indicating pathological changes during NAFLD/NASH progression. Circulating 7-Keto-DCA level was closely related to advancing fibrosis and ballooning, and serum level of conjugated primary BAs were associated with severity of lobular inflammation and fibrosis in NASH [9]. The increased percentage of circulating primary conjugated BAs and decreased unconjugated BAs served as crucial predictors to assess the fibrosis stage of NASH [7]. The increased ratio of conjugated CA in circulating BAs pool is not only closely related to the progression of NASH, but may also contribute to disease progression through CA-medicated inflammation, insulin resistance, and hepatic steatosis [10]. There are controversial results regarding BAs profile in NAFLD/NASH, which may be due to sample size, individual biological diversity, and technical performance between various studies. In addition, the ratio between specific BAs pairs may play a more critical role than single or total BAs in predicting NAFLD/NASH progression [11].
As a BAs-activated nuclear receptor, FXR is downregulated during the development of liver fibrosis, while activation of FXR significantly improves liver fibrosis and inflammation via antagonizing Hepatic Stellate Cell (HSC) activation and the NLRP3 inflammasome mediated lipid aggregation and macrophage activation in NASH models [8,12]. Obeticholic Acid (OCA), a semi-synthetic derivative of CDCA and a potent FXR agonist, ameliorated hepatocellular ballooning and lobular inflammation in NASH subjects in a phase 3 NASH clinical trial [6]. MET409, a structurally optimized nonbile acid FXR agonist, markedly reduced hepatic fat content in a 12-week NASH clinical trial, though with ALT increase [13].
The pathology of cholestatic liver diseases, especially Primary Biliary Cholangitis and Primary Sclerosing Cholangitis (PBC and PSC), progress from immune cells-elicited inflammatory injury of interlobular and large bile ducts to cholestasis. Impaired bile acids transport contributes to cholestasis development. Circulating BA profiles may respond to the alteration in the total BAs pool in PBC subjects. PSC patients with hepatic decompensation have higher circulating concentrations of unconjugated and conjugated BAs [1]. In addition, PSC patients showed exhaustion of fecal secondary BAs, particularly DCA, LCA, and GLCA [14]. The opposite relationship between secondary bile acids content and cholestatic severity suggests the involvement of host microbiota-regulated bile acid metabolism in PSC development. Indeed, alkaline phosphatase level was ameliorated in PSC patients who received fecal microbiota transplantation based on microbiome therapies, suggesting a promising way of correcting BAs dysregulation and PSC [15].
TGR5 acts as a bile duct protective factor evoking proliferation and apoptosis resistance to alleviate bile acid toxicity under cholestatic conditions [16]. The TGR5 expression was decreased in the bile ducts with either PSC livers or the genetic mouse model of PSC, likely predisposing biliary epithelial cells to BAs toxicity and cholestatic damage and subjected the host more vulnerable to the progression of PSC and PBC [17].
Ursodeoxycholic Acid (UDCA), a hydrophilic BA converted from CDCA, is among the first-line therapeutics for PBC and an alternative option for PSC. UDCA therapy reduced the relative risk of liver transplantation or death among patients with PBC and effectively prolonged liver transplantation-free survival for PBC patients [18]. Although UDCA therapy amplified the total BAs pool, toxic conjugated and unconjugated CA and CDCA were largely decreased in PSC patients with UDCA treatment [1]. Unfortunately, the serum concentrations of toxic LCA were elevated in patients treated with UDCA, likely produced by host microbiota in the intestine of PSC patients. However, neither vancomycin-mediated microbiota elimination nor UDCA improved the progression of PSC [19]. Given the limitation of UDCA in cholestasis treatment, PBC patients can be treated with OCA, which decreases hepatocyte exposure to toxic bile acids by promoting the transport of endogenous conjugated bile acids from hepatocytes into biliary canaliculi [5].
Low-Level Viremia (LLV) of Chronic Hepatitis B (CHB) following antiviral therapy is a vital risk factor for the progression of Hepatocellular Carcinoma (HCC), especially for patients with cirrhosis. Serum bile acids have been described as prognostic markers predicting antiviral treatment outcomes [20]. Consistently, our study showed a significant association between DCA content and the occurrence of LLV, which was more evident in patients maintained over 54 weeks of antiviral therapy with nucleoside analogs, suggesting the involvement of DCA in LLV development and HBV maturation. HBV preS1 domain competitively binds to NTCP, inhibiting the uptake of the conjugated BAs, and the expression of BAs synthesis genes markedly increased in the liver tissue of HBV-infected human chimeric mice and liver biopsy samples from patients chronically infected with HBV, likely causing enhanced serum BAs concentration [21]. CHB patients showed a low ratio of unconjugated to conjugated BAs, possibly due to NTCP-mediated transport of conjugated BAs resulting from HBV infection [2].
Alterations of the BAs pool were associated with dysregulation of the gut microbiome in the CHB patients with moderate to advanced fibrosis, who exhibited higher total and primary BAs in circulation, and decreased bacteria genera abundance and fecal secondary BAs [22]. Our study revealed that HBV DNA levels and hepatic DCA levels were reduced following antibiotic treatment in HBV transgenic mice, suggesting the potential association between the intestinal microbiota-produced DCA and HBV maturation in vivo. It has been reported that inhibiting DCA production or reducing the gut microbes has been shown to block liver cancer development in obese mice [23]. A recent study suggested that OCA exerted postentry functions to suppress HBV infection by potentially altered bile salt metabolism, which is supported by the evidence that OCA does not affect HBV replication in HBV genome integrated stable cells, whereas OCA impeded the production of HBV DNA, mRNA and protein and reduced cccDNA in HepaRG cells and primary human hepatocytes [24]. Therefore, the potential mechanism of the OCA effect on HBV antiviral activity may be mediated by bile acid metabolism in addition to influencing virus entry and postentry process. These results suggested that BAs metabolism is likely involved in the HBV maturation process and life cycle.
BAs homeostasis is intimately mediated by FXR signalling and BAs transporters in liver and intestinal tract under physiological conditions. Abnormal BAs metabolism plays a significant role in the pathogenesis and progress of liver and biliary disease (Figure1). Hepatic BAs synthesized from CYP7A1-mediated cholesterol conversion are secreted into the bile via BSEP. BAs are reabsorbed in the ileum via ASBT and transported back to the liver via enterohepatic circulation. NTCP regulates the reabsorption of conjugated BAs. In hepatocytes, FXR upregulates BSEP and the transcriptional repressor SHP, suppressing CYP7A1 and NTCP transcription. In the enterocytes, BAs-activated FXR upregulates OSTα/OSTβ, resulting in BAs expulsion into portal blood. Intestinal FXR evokes FGF15/FGF19 that circulates to the liver and combine with its receptor FGFR4, subsequently suppressing BA synthesis. Liver disease causes abnormal BAs metabolism. HBV infection influences NTCP-mediated transport of conjugated BAs. Downregulation of FXR reverses its antagonism to HSC, promoting the development of NASH and liver fibrosis. Host microbiota-BAs crosstalk is involved in PSC development. Much remains to be discovered regarding the specific mechanisms of BAs involved in the etiopathogenesis of NAFLD/NASH, cholestatic liver disease, and HBV-related liver disease. The connection between BAs and disease development is not monodirectional but highly intricate. Recent studies further discuss whether the alterations of abundant BAs profiles are a disease outcome, versus an originating cause.
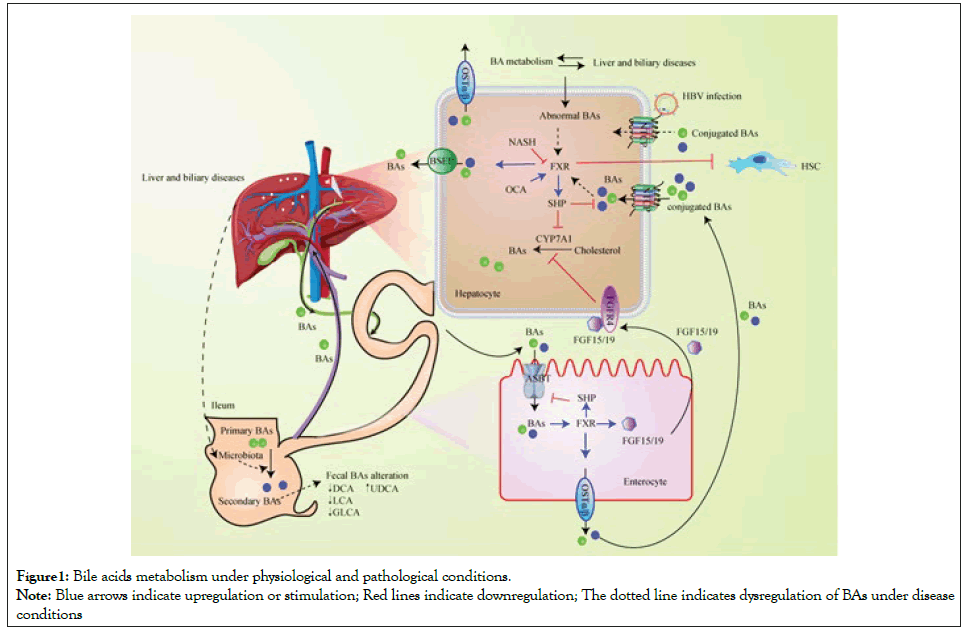
Figure 1:Bile acids metabolism under physiological and pathological conditions.
Note: Blue arrows indicate upregulation or stimulation; Red lines indicate downregulation; The dotted line indicates dysregulation of BAs under disease
conditions
This mini-review summarized recent studies on BAs in regulating non-alcoholic fatty liver disease, cholestatic liver disease, and HBVrelated liver disease. Although BAs and derivatives, such as UDCA and OCA, have already entered clinical trials for liver and biliary diseases, there is still no effective medical therapy available for these diseases. Therefore, it is urgently needed to better understand the BAs regulated pathogenesis to develop safe and more effective therapies.
Financial support statement: This work was supported by the National Natural Science Foundation of China grant 82073676; Beijing Municipal Natural Science Foundation and Beijing Municipal Education Commission grant KZ202010025037 and National Natural Science Foundation of China-the Chinesisch- Deutsches Zentrum füer Wissenschaftsförderung grant C-0012.
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
Citation: Gao YX, Ning Q, Jiang M, Chen D (2022) The Role of Bile Acids in the Liver and Biliary Diseases. J Cell Sci Therapy. 13:341.
Received: 04-Feb-2022, Manuscript No. JCEST-22-15796; Editor assigned: 08-Feb-2022, Pre QC No. JCEST-22-15796 (PQ); Reviewed: 18-Feb-2022, QC No. JCEST-22-15796; Revised: 25-Feb-2022, Manuscript No. JCEST-22-15796 (R); Published: 04-Mar-2022 , DOI: 10.35248/2157-7013-22.13.341
Copyright: © 2022 Gao YX, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.