Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Review Article - (2022)Volume 12, Issue 5
Parkinson’s Disease (PD) is the second most prevalent neurodegenerative disease affecting every 150 out of 100,000 individuals worldwide. PD is characterized by a resting tremor, rigidity and bradykinesia; all displaying asymmetry. Most cases of clinical PD are caused by the degradation of dopaminergic neurons in the midbrain, specifically in the substantia nigra. Death of dopaminergic neurons in this region has been linked to increased oxidative stress and free radical damage. Evidence also supports dopaminergic neuron death caused by a deficiency in mitochondrial complex I which decreases energy production in the substantia nigra of the brain. Other studies have shown that nigral cell death accompanied with gliosis is exacerbated in dopaminergic neurons due to abnormal calcium ion handling. Degradation of dopaminergic neurons is initiated by the formation of protein aggregates known as lewy bodies.
Parkinson’s disease; Gut microbiota; Gastrointestinal system; Mutation
Parkinson’s Disease (PD) is caused by degradation of dopaminergic neurons in the substantia nigra of the midbrain, from lewy body formation made of α-synuclein. Treatment is limited to suppression of symptoms in motor stages of PD instead of early preventive measures. This raises the question of what options can be taken to limit the progression of Parkinson’s disease prior to motor-phase entry [1-3].
Lewy bodies are collections of protein filaments comprised of ubiquitin and α-synuclein. Alpha-synuclein is an intrinsically disordered protein which lacks a stable three-dimensional structure and is characterized by structural plasticity and conformational adaptability. Thus, it is capable of binding to other molecules easily. It has a variety of normal roles including synaptic function, regulating the size of presynaptic vesicles, Soluble N-ethylmaleimide-Sensitive Factor Activating Protein Receptor (SNARE) protein assembly and docking of synaptic vesicles to presynaptic membranes. When α-synuclein is overexpressed, it can form lewy bodies. These aggregates can lead to protein accumulation in substantia nigra dopaminergic neurons and therefore increase risk of cell death. One theory to explain protein aggregate-induced neuron all cell death called the “black hole” effect, states that proteins within the neuron attract, aggregate and clog the cytoplasm which causes the impairment of intracellular function and irrevocable damage to the neuron. This is detrimental because the degeneration of these dopaminergic neurons, causes common Parkinson’s characteristics including rigidity, tremors, abnormal gait and posture [4,5].
Parkinson’s etiology
Parkinson’s disease cannot be narrowed down to one specific etiology. Some environmental agents have been identified which may cause PD. Neurotoxin 1-Methyl-4-Phenyl-1,2,3,6-TetrahydroPyridine (MPTP) is currently the only known environmental agent that can cause PD with symptom presentation only two weeks after exposure [1]. Environmental agents including herbicides and pesticides have also been associated with an increased risk of PD. Genetics may play a role in the development of PD, with several studies demonstrating that first degree relatives of Parkinson’s patients have a 2-3 fold increased risk of developing the disease. Mutations on the α-synuclein gene located on chromosome 4, as well as mutations in the parkin gene on chromosome 6, have been linked to increased risk of PD in autosomal dominant and recessive patients. Both autosomal dominant and recessive patients with these mutations exhibit similarities in the early onset of disease, however these patients lack the hallmark PD symptom of a resting tremor. Mutations on chromosome 2 have been shown to cause autosomal dominant PD but have been associated with a lesser ability to cause the disease which is why chromosome 2 mutations are most often related to a more sporadic form of PD. There are treatments available for patients affected with Parkinson’s disease however, they are primarily symptom management therapies to decrease the severity of motor symptoms associated with the disease.
Current treatment of Parkinson’s disease
There is a lack of full understanding regarding risk factors which cause the development of PD. Therefore, treatment is limited to suppression of symptoms in later stages of progression. Levodopa is the most used treatment in mitigating the symptoms of PD [1]. It is a dopamine precursor, converted to dopamine within presynaptic neurons and is typically paired with a non-specific decarboxylase inhibitor in order to reduce peripheral side effects and increase absorption of the drug into the CNS. Levodopa may also be administered with Catechol-O-Methyltransferase (COMT) inhibitors which work similarly to decarboxylase inhibitors by increasing the penetration of levodopa into the brain and improve subsequent conversion to dopamine.
Surgical interventions have been suggested to help mitigate Parkinson’s symptoms. These include thalamotomies and pallidotomies. A thalamotomy is associated with a decrease in thalamic activation, which appears to be successful in controlling resting tremors experienced by PD patients. A pallidotomy, which is done at the internal area of the globus pallidus, would cause decreased inhibitory outflow from the basal ganglia. This is associated with improved hemiballism causing an increase in sudden movement as well as the elimination of dyskinesias (abnormal motor movements) caused by PD [5].
An increased knowledge of prodromal Parkinson’s can be useful in identifying potential PD patients earlier, and thereby administering early protective treatment prior to PD pathogenesis. Protective therapy is one approach to the treatment of PD by preserving neural structure and function of the dopaminergic neurons. One protective therapy found to be very effective since the 1990s is selegiline which is a neuroprotective agent. Neuroprotective agents are capable of blocking the apoptotic process due to a transcriptional effect. Selegiline is a monoamine oxidase B inhibitor which increases the duration of dopamine in the synapse and possesses anti-apoptotic properties. Evidence suggests that the use of selegiline could potentially delay additional pharmacological treatment for up to one year in early PD patients. It is more useful in patients in the early stages of PD compared to those in late PD because those in late PD show greater amounts of dopaminergic neuronal death, limiting the efficacy of selegiline.
The bidirectional pathway of Parkinson’s disease
The dual-hit hypothesis: One hypothesis for the development of Parkinson’s disease has been the concept that the disease originates from synapses in the Peripheral Nervous System (PNS) which then invades the brain by retrograde axonal transport via the vagus nerve [6]. This would be considered a PNS-first phenotype where pathology begins in the periphery and then spreads to the brain.
Retrograde axonal transport supports the dual-hit hypothesis and is reinforced by evidence including PNS aggregation of the protein α-synuclein in pre-PD. The proposed mechanism for this hypothesis includes two pathways for initiation of the pathology. One is anterograde by the olfactory pathways and the other is retrograde via the enteric plexus and preganglionic vagal fibers. Both pathways lead to abnormal α-synuclein expression, Lewy body formation and substantia nigra damage. The olfactory mechanism suggests that the brain and olfactory nerve are directly connected such that if a neurotoxic agent were to cause PD, it would have easy access to dopaminergic neurons in the brain [7].
The gut-brain axis: Development of PD has been supported by the idea of a “gut-brain axis” where there is an association between the CNS and the Enteric Nervous System (ENS), allowing the brain and the gut to be in communication. Activity in the brain can be influenced by gut microbes as well as Gastrointestinal (GI) inflammation and immune responses [8]. The most direct pathway between the brain and gut is the vagus nerve which starts in the Dorsal Motor Nucleus of the Vagus (DMV) in the medulla and extends into the abdomen and visceral tissue evidence shows the vagus nerve can act as a conduit which could allow material to pass into the brain directly from the intestine. Studies have demonstrated that PD patients experience gut inflammation prior to the pathogenesis of the disease. Which supports the idea of gut-originating, inflammation-driven Parkinson’s disease illustrated in Figure 1 [8]. Gut microbiota are negatively impacted by inflammation and immune responses in the gut leading to an increase in intestinal permeability. An increase in inflammation causes α-synuclein overexpression and aggregation, which can increase α-synuclein transport along the vagus nerve retrograde to the CNS. Intestinal inflammation could also cause systemic inflammation, leading to increased permeability in the blood-brain barrier. Increased α-synuclein and/or systemic gastrointestinal inflammation could increase neuroinflammation leading to neurodegeneration of dopaminergic neurons and causing PD.
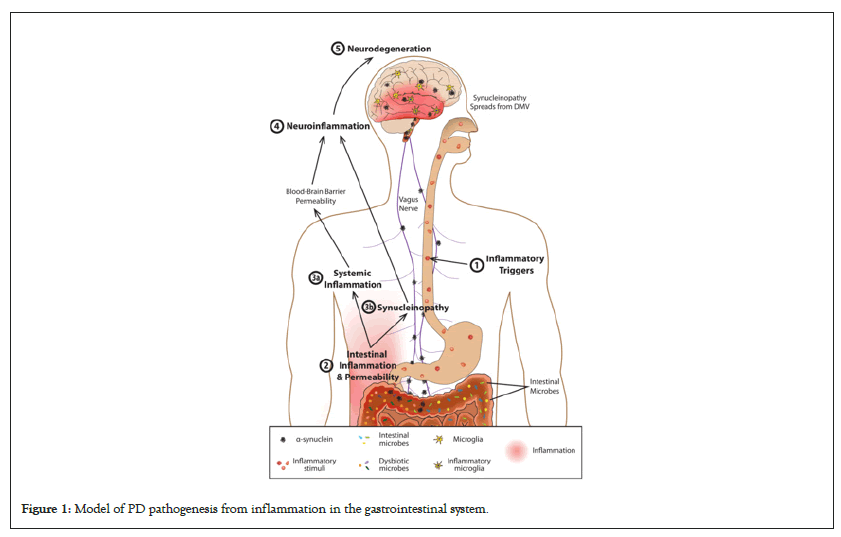
Figure 1: Model of PD pathogenesis from inflammation in the gastrointestinal system.
The gut-brain axis mechanism for PD pathology has a sequence to produce neurodegeneration. Initially, an inflammatory trigger is introduced in the gut which could be a GI infection or a toxin produced by the gut microbiota [8]. Low-level inflammation develops in the gut which is sustained and can harm healthy microbiota in the gut as well as increase intestinal permeability. Microbe-produced toxins can leak into surrounding systemic tissue via increased permeability, causing body-wide pathological immune responses. This systemic inflammation may also change permeability of the blood brain barrier. Inflammation in the gut has been linked to over-expression of peripheral α-synuclein which can also spread to surrounding tissue. From the periphery, α-synuclein travels via retrograde transport along the vagus nerve to the CNS. Many studies have shown that PD pathology begins in the dorsal motor nucleus of the vagus nerve in the CNS. From the DMV, inflammation and synucleinopathy could occur in other brain regions including the substantia nigra and cause degeneration to dopaminergic neurons. Following substantial neural degeneration, motor and clinical symptoms of PD will appear [8].
One observational study in baboon monkeys investigated pathological Lewy body development following either enteric or nigrostriatal injection of post-mortem synuclein-containing tissue [9]. The post-mortem brain tissue used contained Lewy body aggregations in up to 90% of the samples. Sample tissue from the monkeys were measured two years after injection by Tyrosine Hydroxylase (TH) immunohistochemistry which identifies the number of dopaminergic neurons in the CNS. Two years after injection, all the monkeys displayed a loss in dopaminergic neurons at their site of injection whether it was in the CNS or ENS. It was also noted that injections of lewy bodies into the ENS led to dopaminergic neuron loss in the striatum which supports the gut-brain pathway hypothesis for development of PD. Lewy body accumulation in the ENS was also present following striatal injection, but accumulation was lower than in the striatum after ENS administration. Based on this data, it can be seen that PD pathogenesis can have a potential to originate in the gut from increased synucleinopathy and trafficking of synuclein to the brain via retrograde transport.
The premotor phase of Parkinson’s disease
Non-motor symptom presentation: A major biomarker of PD is the onset of non-motor symptoms which tend to indicate a patient’s entry into the premotor phase of Parkinson’s. This preclinical stage also been associated with dysfunction of the gut microbiota which may be associated with non-motor symptoms [10]. Prior to the development of motor symptoms, clinically relevant Non-Motor Symptoms (NMS) can include constipation, smell or taste loss, fatigue and nightmares associated with rapid eye movement sleep behavior disorder. Most of these symptoms occur 2 years-10 years before the onset of motor symptoms which can be a key point of therapeutic intervention. As PD is a progressive disorder without cure, a goal of treatment would be to keep patients in the premotor phase for as long as possible before motor symptoms develop. Detection of biomarkers and their subtypes are outlined in Table 1 [10].
| Pre symptomatic biomarker type | subtype | Identifiable characteristics |
|---|---|---|
| Clinical biomarkers | NMS (appears 10 years before motor symptoms) | Constipation, dream enacting behavior, frequent nightmares, excessive day time sleepiness, post prandial fullness, tremor |
| NMS (appears between 2-10 years before motor symptoms) | Smell loss, mood disturbances, excessive sweating, fatigue, pain, constipation, hypotension, urinary dysfuntion, erectile dysfunction | |
| Biochemical biomarkers | Metabolic factors | Cholesterol level |
| Neurotrophic factors | High IGF-1,Low vitamin D | |
| Imaging biomarkers | Oxidative stress | High uric acid level |
| Nuclear imaging | Nigrostriatal degeneration | |
| Transcranial sonography | Substantia nigra hyperechogenicity | |
| Magnetic resonance imaging | Reorganization of corticostriatal circuits in mutations |
Note: NMS: Non Motor Symptoms; IGF: Immunoglobulin F.
Table 1: Characteristics of pre-symptomatic stage biomarkers in Parkinson’s disease.
Constipation as a Parkinson’s disease non-motor symptom: Constipation occurs from a delay in movement in the small and large intestine [10]. It can present in PD patients up to 16 years-24 years prior to the diagnosis of PD and well before motor symptoms appear. A study conducted by Abbott et al., showed that middle-aged men who had less than one bowel movement daily had four times increased risk for a PD diagnosis compared to those men with normal bowel function [11-13]. Constipation is also twice as common in patients who developed PD than normal, age-matched, healthy patients.
Gut-brain axis in Parkinson’s disease pathogenesis: Association of the gut-brain axis in with non-motor symptom development: The gut-brain axis is putatively responsible for many processes which can affect cognitive function. Research has linked cognitive disorders including depression and anxiety with dysfunction in the gut microbiota. The synthesis of enzymes producing dopamine is regulated in part by the gut microbiota via the gut-brain axis. Constipation, a major NMS of PD, has been associated with the deposition of α-synuclein in the gastrointestinal system causing local inflammation, increased intestinal permeability and oxidative stress. Colonic biopsies have shown, compared to normal controls, PD patients have increased messenger Ribonucleic Acid (mRNA) expression of proinflammatory cytokines. This shows that the intestinal microbiota needs to be balanced to maintain a proper gut environment. GI microbiota are important to prevent intestinal and systemic inflammation which can lead to disturbances in the Central CNS as well as α-synuclein propagation. Systemic inflammation can be caused by gastrointestinal inflammation leading to increased permeability of the blood brain barrier. Together, these data provide a potential mechanism for retrograde transport of α-synuclein to the CNS which further supports the role of the gut-brain axis as a mechanism for PD development.
Effect of the gut microbiome in Parkinson’s disease
Pathogenic gut bacteria in Parkinson’s disease development: Inflammatory bacteria can cause Small Intestinal Bacterial Overgrowth (SIBO) which is associated with Parkinson’s disease [10]. In a study analyzing fecal samples from a group of 72 PD patients, 25% higher levels of Enterobacteriacea were shown along with lower levels of Prevotellacea. This was of concern because Prevotellacea promotes a healthy intestinal environment by breaking carbohydrates into short chain fatty acids and acts as an anti-inflammatory commensal gut bacteria. A decrease in Prevotellacea is associated with a decrease in micronutrients that also aid the survival of other gut microbes which are linked to dopamine production in the gut. Increased levels of Enterobacteriacea was also of concern because this bacteria is very commonly overgrown in the gut causing inflammation and is one of the main overexpressed bacteria in inflammatory bowel diseases. Another bacteria associated with impairment of motor function is Helicobacter pylori. H. pylori is associated with patients in the motor phase of PD and can limit the absorptive capabilities of levodopa, causing worsening of PD symptoms due to insufficient systemic concentrations.
Cyanobacteria, found in lower quantities in the gut, is known to produce β-N-Methyl-Amino-L-Alanine (BMAA) which is an excitotoxin found in the brains of many PD patients [12]. BMAA works by activating a metabotropic glutamate receptor which leads to decreased production of a major antioxidant, glutathione. BMAA is also involved in protein misfolding, which has implications for the misfolding and aggregation of the α-synuclein protein comprising lewy bodies in PD. E. coli can produce salsolinol which has been shown to aid the development of PD Salsolinol is produced by E. coli in the presence of dopamine and was enhanced by alcohol consumption. Altered gut microbiota in some patients with PD was recorded by Villageliu et al., and derived data can be seen in Table 2 [14].
| Altered bacteria | Overexpressed ↑ | Function in the gut |
|---|---|---|
| Under-expressed ↓ | ||
| Prevotellacea | ↓ | Anti-inflammatory |
| Enterobacteriaceae | ↑ | Inflammatory |
| Lactobacillus | ↓ | Neuroprotection |
| Roseburia | ↓ | Anti-inflammatory |
| Coprococcus | ↓ | Anti-inflammatory |
| Blautia | ↓ | Anti-inflammatory |
Table 2: Altered microbiota in patients with PD.
Beneficial gut bacteria in Parkinson’s disease development: There are many beneficial gut bacteria which can have implications in selection of appropriate probiotic treatment for patients in the pre-motor phase of PD who are experiencing gastrointestinal dysfunction. One important biomarker for pre-motor PD was decreased Prevotella in the gut. Prevotella is associated with a decreased synthesis of mucin, a molecule which is associated with maintaining gut integrity. Decreased Prevotella levels may therefore cause increased gut permeability [14]. Prevotella is also associated with the release of hydrogen sulfide which has a neuroprotective role for dopaminergic neurons as demonstrated with rat and mouse models of PD. A decrease in Lactobacilliceae in the gut has also been associated with PD patients. Lactobacilliceae is associated with the hormone ghrelin, known to be involved with neuroprotection of nigrostriatal dopamine as well as impacting eating behavior. Bacillus spp. is another beneficial bacteria as it produces dopamine in the gut and accounts for most of the total dopamine levels in the body. Anti-inflammatory bacteria from the genus Roseburia, Coprococcus and Blautia have been found in much lower levels of PD patient fecal samples indicating a potential role in dysregulation of the gut-brain axis and PD symptom presentation in Table 2 [15,16].
The role of the appendix: The appendix is included with gut-associated lymphoid tissue and is rich in immune cells as well as acting as a storage for gut bacteria [17]. It is connected to the end of the cecum as a narrow finger-like pouch. Recent evidence linked inflammation in the appendix with development of PD. The appendix has an abundance of α-synuclein in the aggregated form, leading to potential for creating lewy bodies. The appendix is also innervated by the vagus nerve and therefore could serve as a potential route of the α-synuclein aggregates to the brain. Certain studies have demonstrated that early-life removal of the appendix in patients up to 52 years of age was associated with a reduced risk for PD. In the prodromal phase of PD, it is also shown that α-synuclein aggregates are found in greatest amount in the appendix compared to other parts of the GI system. Research has demonstrated that α-synuclein aggregate density is greatly increased around the nerve terminals of the myenteric plexus of the vagus nerve in the appendix, which further supports the idea of gut-brain α-synuclein propagation via the vagus nerve [17].
Inflammatory bowel diseases: Implications for the nervous system/ Parkinson’s disease development
Probiotic treatment for inflammatory bowel diseases may provide a great preventative measure as well as a mitigating factor for disorders of the gut. Probiotic function includes inhibition of growth of pathogenic bacteria, improving epithelium function of the gut and mitigation of immune responses in the host. Probiotics have shown capabilities in enhancing mucosal integrity, nourishing cells of the GI system, inhibiting cell apoptosis and improving bowel dysmotility. Probiotics constitute a cornerstone of therapy for some inflammatory bowel diseases and may represent a novel therapeutic avenue for pre-motor PD [18,19].
Ulcerative colitis: Ulcerative Colitis (UC) is a chronic disorder of the intestinal tract associated with symptoms such as malnutrition, abdominal pain and diarrhea [20]. A decrease in anti-inflammatory bacteria and an increase in inflammatory gut bacteria has been observed in patients with UC. Gut microbiota alterations are heavily involved in the development and progression of UC and has even been shown to induce neurological disorders in these patients, including anxiety and depression via the gut-brain axis due to an inflammatory gut response. This has major implications on the potential benefit of probiotic therapy to mitigate inflammatory responses in the gut and thereby prevent cognitive dysfunction. A recent study has shown that there is a 2-fold decrease in Roseburia intestinalis in patients with UC, which decreases metabolic function of the host. Similar to UC, Roseburia bacteria is also decreased in patients with PD (Table 2). It was hypothesized in the article by Xu et al., that restoration of Roseburia via probiotics may have protective effects for the nervous system [20]. Administration of R. intestinalis improved depressive-like symptoms in rats with UC and was also associated with lower levels of inflammatory markers IL-6 and IL-7. Decreased inflammation provides insight to benefits of treating inflammatory bowel diseases with probiotics to lessen symptom severity and decrease potential future neurodegenerative effects.
Irritable Bowel Syndrome (IBS): Irritable Bowel Syndrome (IBS) is a disorder associated with changes in bowel habits such as motility, gut hypersensitivity and increased inflammation [19]. Bi-directional communication is known to occur between the ENS and the CNS in IBS and is increased with disturbances to gut homeostasis. As in PD, IBS is associated with changes in gut microbiota and disruption of the intestinal barrier permeability. Small Intestinal Bacterial Overgrowth (SIBO) is also associated with IBS where a bacterial dysbiosis in the gut causes gastrointestinal symptoms such as constipation and pain. This may have implications to the possible cause of PD by SIBO in the gut, supporting the hypothesis that a dysfunction in gut microbiota can have potential causal effects in the pathogenesis of PD. SIBO in IBS is associated with the release of certain inflammatory molecules such as histamine and interleukins which have been shown to increase gut permeability and inflammation. Patients with IBS have poor gut motility, causing pain and constipation from SIBO which is a proposed etiology for the disorder. Similar to PD, prodromal symptoms for IBS include constipation which is mitigated by the early use of probiotic therapy.
Treatment with Lactobacillus, Bifidobacterium and Propionibacterium species in IBS has shown to be effective for improving gut function and decreasing inflammation. UC symptoms also decrease with probiotic treatment. Treatment with certain strains of E. coli have been beneficial towards the mitigation of symptoms in UC, by decreasing gut permeability, inflammation and movement of pathogenic α-synuclein to the CNS via the gut-brain pathway. Gut bacteria alterations in inflammatory bowel diseases show similarities to those over and under-expressed in PD. This has implications for use of probiotic therapy in PD to effectively mitigate pre-motor symptoms of PD and keep patients in the pre-motor phase for a longer period [21].
Implications for probiotic therapy on Parkinson’s disease treatment
Potential use of probiotics in Parkinson’s disease prevention: With the understanding that pre-motor PD involves symptoms such as constipation and gastrointestinal pain, there may be potential for the use of probiotic therapy in PD to help increase the time before onset of motor symptoms. Dopaminergic neuron loss is associated with gastrointestinal dysfunction and decreased motility leading to constipation [22]. PD patients have a different composition of bacteria in the gut versus healthy individuals, with altered levels of Prevotella, Lactobacillus and Enterobacteriacea. Probiotics have the potential to mitigate PD by producing a decrease in cytokine production to reduce gut inflammation and permeability. Experiments conducted by Magistrelli et al., aimed to investigate the in vitro effects of probiotics in PD patients compared to healthy patients. They assessed levels of cytokines released in the gut and the ability to restore gastrointestinal membrane function after probiotic therapy [22]. Six different probiotic strains were used including: Lactobacillus salivarius (LS01), Lactobacillus plantarum (LP01), Lactobacillus acidophilus (LA02), Lactobacillus rhamnosus (LR06), Bifidobacterium animalis (BS01) and Bifidobacterium breve (BR03). Cytokine release by peripheral blood mononuclear cells in the gut was measured before and after probiotic therapy and is shown in Figure 2.
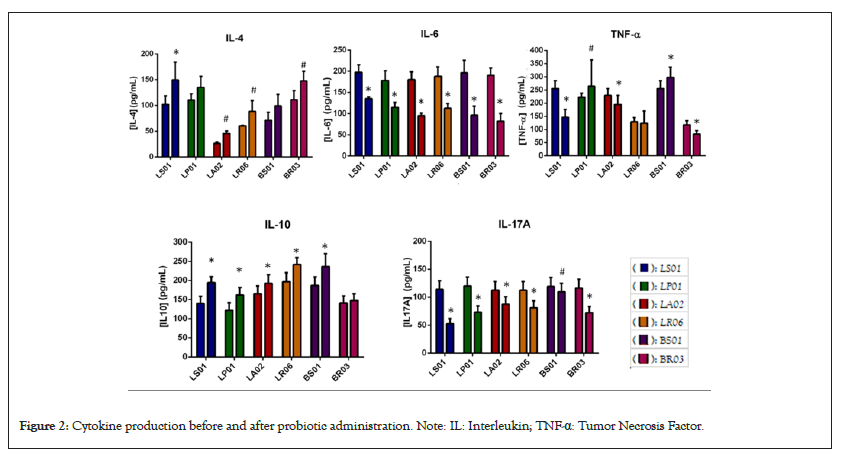
Figure 2: Cytokine production before and after probiotic administration. Note: IL: Interleukin; TNF-α: Tumor Necrosis Factor.
These data suggest that probiotic strains have the capability to modulate inflammatory cytokines and protect the gut from increased permeability. The authors also concluded that probiotic therapy is beneficial in aiding levodopa absorption as demonstrated by a 50% increase of levodopa concentration in the gut following therapy [23-27].
PD patients are found to have altered levels of Prevotella, Lactobacillus and Enterobacteriacea compared to patients without the disease. Probiotic strains that were identified to have protective effects on the gut, include Lactobacillus salivarius, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium animalis, and Bifidobacterium breve. PD patients have a decrease in Prevotellacea associated with less micronutrients that aid the survival of other gut microbes producing dopamine. Helicobacter pylori is associated with patients in the motor phase of PD and can limit the absorptive capabilities of Levodopa.
Promoting good gut health
There is a good amount of evidence which supports the idea of a bi-directional gut-to-brain pathway as well as a role for gut dysfunction in both pre-motor and clinically impaired PD. Healthy gut microbiota can help prevent or even treat a variety of gut and neurological dysfunctions including IBS. Our hypothesis is that α-synuclein can propagate from the ENS to the CNS due to increased inflammation and gut permeability caused by an impaired or altered gut microbiota. Factors such as SIBO, constipation and inflammatory bacterial by-products found from PD patients indicate that bowel function heavily relies on good gut health. Pro-inflammatory gut environments caused by a dysbiosis of bacteria can lead to a dysfunctional blood brain barrier, leading to movement of α-synuclein into the brain transported via the vagus nerve from the Enterprise Nervous System (ENS). Adjusting the gut microbial environment can allow for improved absorption of medications for PD such as levodopa. Early biomarkers such as salsolinol and bacteria levels found in PD patient stool samples can help with identification and potential treatment of prodromal/pre-motor PD. Treatment with probiotics using beneficial bacteria as a neuroprotective mechanism in PD as well as regulation of the gut in pre-motor PD and other inflammatory bowel disorders represents a novel treatment modality for PD.
Keeping patients in pre-motor Parkinson’s disease phase
Pre-motor PD presents with a variety of symptoms which can be useful biomarkers to allow for earlier diagnosis. Early identification is important to keep PD patients in this phase and prevent further progression to the motor phase of the disease. Non-motor GI symptoms such as constipation should be highly considered in diagnosis as well as analysis of SIBO, α-synuclein and intestinal barrier degradation early in treatment [9]. It is important that early diagnosis be conducted to slow the disease course and to keep PD patients in the pre-motor phase. Keeping patients in the pre-motor phase of PD can prolong their time before the onset of motor symptoms arise. This is important in order to maintain CNS function as a preventative measure for the long term because many PD patients develop neurodegenerative disorders in the late stages of PD. Detecting patients in the pre-motor phase can give a more efficient time frame for drugs to be administered to the patient as a neuroprotective mechanism. Patient quality of life could also be improved if they are kept in this pre-motor phase. This would prevent patients from developing resting tremors as well as shuffled movement and rigidity.
Based on the current information regarding α-synuclein propagation from the gut to brain, if that mechanism is slowed, less dopaminergic neuron death could occur leading to decreased motor symptoms. This pathway is a direction for future study as to the specific mechanisms and correlations between a decrease in α-synuclein propagation via the bi-directional gut-brain axis and the specifics of time to when motor symptoms may appear in PD patients. Considerations such as how long probiotic treatment would need to be administered, whether it would be continual through PD progression or for a certain period in the pre-motor phase is also something that can be investigated further. Although there may not be a unanimous idea of the most beneficial PD treatment method, there appears to be an alternative route for slowing PD progression by administration of probiotic therapy as soon as pre-motor symptoms begin to appear and even as a general concept to promoting good gut health.
Gut microbiota are negatively impacted by inflammation, which in turn increases the intestinal permeability and influences the retrograde transport of α-synuclein to the central nervous system, leading to its aggregation seen in PD. There is evidence that dopaminergic neuron loss results from a deficit in mitochondrial complex I, which lowers the brain's substantia nigra's capacity to produce energy. Previous research has demonstrated that aberrant calcium ion handling in dopaminergic neurons exacerbates nigral cell death concomitant with gliosis. The production of protein clumps called lewy bodies is what starts the degeneration of dopaminergic neurons. Hence, probiotic treatment may present a novel therapeutic avenue for pre-motor PD by enhancing mucosal integrity, nourishing cells of the gastrointestinal system, inhibiting apoptosis and improving bowel motility.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Katz A (2024). The Role of the Gastrointestinal System in the Progression of Parkinsonâ??s Disease: A Novel Approach to Elongating Patient Time in Pre-motor Parkinsonâ??s Disease. 12:358.
Received: 09-Aug-2024, Manuscript No. JPH-24-33458; Editor assigned: 12-Aug-2024, Pre QC No. JPH-24-33458 (PQ); Reviewed: 26-Aug-2024, QC No. JPH-24-33458; Revised: 02-Sep-2024, Manuscript No. JPH-24-33458 (R); Published: 10-Sep-2024 , DOI: 10.35248/2329-8901.24.12.372
Copyright: © 2024 Katz A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.