Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2024)Volume 9, Issue 5
Background: Angiogenic markers serve as crucial indicators of Adverse Pregnancy Outcomes (APRO). However, the variation in specific cut-off values used to assess APRO risk complicates their clinical utility. This study aims to identify the most predictive angiogenic marker or combination thereof for Adverse Maternal Outcomes (AMO), determining the optimal cut-off point for highest accuracy.
Methods: This observational retrospective cohort study utilized hospital medical records. We categorized singleton pregnancies (21-40 weeks gestation, n=60 each) into three groups based on sFlt-1/PlGF ratio levels: High (≥ 655), Intermediate (≥ 85 to <655) and Low (<85). Binary logistic regression was employed to identify the best predictors of AMO. Receiver Operating Characteristic (ROC) analysis was used to compare detection rates and determine the optimal cut-off.
Results: Significant differences were observed among the sFlt-1/PlGF groups (High>Intermediate>Low) for systolic and mean blood pressure, angiogenic markers, Aspartate Aminotransferase (AST), Alanine Transaminase (ALT) and AMO (p<0.001). The sFlt-1/PlGF ratio showed the highest Area Under the Curve (AUC) for predicting AMO compared to individual parameters. A cut-off point of 137 for the sFlt-1/PlGF ratio was identified, with no significant difference from the best models obtained. Assuming a pre-test AMO probability of 2%, the negative likelihood ratio was 0.098 and the positive likelihood ratio was 3.11. A negative test result yielded a post-test probability of AMO of 0.2%, while a positive test result yielded 7%.
Conclusion: The sFlt-1/PlGF ratio correlates with AMO severity and surpasses single parameters in predictive accuracy. A recommended cut-off of 137 for the sFlt-1/PlGF ratio is suggested for ruling out AMO in clinical practice.
sFlt-1/PlGF ratio; Placental insufficiency; Adverse maternal outcome; Preeclampsia
sFlt-1: Soluble Fms-like tyrosine kinase; ACOG: American College of Obstetricians and Gynecologists; AO: Adverse Outcome; APO: Adverse Pregnancy Outcome; AUC: Area Under the Curve; FIGO: International Federation of Gynecology and Obstetrics; IUGR: Intrauterine Growth Restriction; NICE: National Institute for Health and Care Excellence; PE: Preeclampsia; PlGF: Placental Growth Factor; ROC: Receiver Operating Characteristic Curve
Preeclampsia and Intrauterine Growth Restriction (IUGR) are closely linked to placental dysfunction. In both conditions, the sFlt-1/PlGF ratio has emerged as a significant surrogate marker, capable of predicting Adverse Pregnancy Outcomes (APRO) [1-11]. Recently, the Food and Drug Administration (FDA) approved the sFlt-1/PlGF test for early preeclampsia to assist in assessing the risk of progression to preeclampsia with severe features within two weeks [12].
Several authors have proposed the use of a continuous scale to study the relationship of sFlt-1/PlGF ratio with APRO [13,14]. However, most authors use specific cut-offs to evaluate the risk of APRO, with values that range from 38 to 1000, including values in between such as 40, 85, 110, 178, 201, 377, 655 and 871 [15-26].
In addition to Advanced Protein Research Organization (APRO), it would be important to identify if the reason for the change in the sFlt-1/PlGF ratio is maternal or fetal, if the sFlt-1/PlGF ratio can predict adverse maternal or fetal events and how the ratio compares with other parameters (clinical or analytical) or models in the prediction of AMO [27-29]. Also some have proposed PlGF to help in the diagnosis of preeclampsia. In this work, we aimed to evaluate with use of the angiogenic markers which combination of factors better predicts the Adverse Maternal Outcome (AMO), as well as, the cut-off yielding the highest accuracy [30-33].
Study design and setting
This observational retrospective cohort study took place at Hospital Universitario Politecnico La FE in Valencia, Spain.
Inclusion criteria
The study included pregnant patients with singleton live fetuses between 21 and 40 weeks of gestation. Patients were categorized based on their sFlt-1/PlGF levels into three groups: High (≥ 655), intermediate (≥ 85 to <655) and Low (<85).
Exclusion criteria
Patients were excluded if there was missing information on maternal or fetal complications in the clinical records. Additionally, patients who transitioned to a lower sFlt-1/PlGF group during the study period were excluded from analysis. The study received approval from the Institutional Review Board of Instituto de Investigacion Sanitaria La Fe (2018/0202) and informed consent was obtained from all participants.
Definitions
Preeclampsia and severe preeclampsia: Defined according to American College of Obstetricians and Gynecologists (ACOG) guidelines [34]. We also included criteria for concomitant hypertension with uteroplacental dysfunction, such as fetal growth restriction [35,36].
Fetal growth restriction
Defined based on FIGO criteria [37]. Risk Factors for preeclampsia and Placental Dysfunction-Related Disorders, as proposed by ACOG and NICE [34,38].
Risk quantification and sFlt-1/PlGF assessment
We established a risk quantification system for preeclampsia based on a point allocation: 1 point for low risk, 2 points for two or more low-risk factors or one high-risk factor, 3 points for diagnosed preeclampsia or one high-risk factor plus one or more low-risk factors and 4 points if maternal adverse outcomes were previously documented, aligning with published estimates. The evaluation of the sFlt-1/PlGF ratio was prompted by clinical suspicion of placental dysfunction disorders [39-42].
Data collection and outcome measures
Data encompassing gestational details-parity, gestational number, maternal ethnicity, age, weight and height-were extracted from hospital records. Adverse Maternal Outcomes (AMO) encompassed maternal mortality, severe preeclampsia and any maternal condition preventing expectant management per ACOG guidelines. Maternal serum levels of sFlt-1 and PlGF were quantified using the Elecsys sFlt-1 and Elecsys PlGF assays on the cobas® electrochemiluminescence immunoassay platform (Roche Diagnostics GmbH, Mannheim, Germany). The sFlt-1/PlGF ratio group assignment was based on the highest recorded ratio during pregnancy [43-46].
Statistical analysis
Tests were selected based on variable normality. ROC analysis was applied to assess parameter utility in predicting AMO, determining AUC for optimal participant classification. Using Youden’s index, a cut-off was established, with sensitivity, specificity and positive and negative likelihood ratios calculated. A binary multivariate logistic regression model was developed to predict AMO, considering maternal characteristics, medical history and biomarkers as predictors. Statistical analyses were conducted using International Business Machine (IBM) and Statistical Package for the Social Sciences (SPSS) Statistics 26 (SPSS Inc.; Chicago, Illinois), with significance set at p<0.05 [47,48].
Participant selection and characteristics
We enrolled a total of 180 women, with 60 participants in each group. The rationale for angiogenic marker assessment is detailed in Table 1, highlighting established preeclampsia as the predominant reason in the intermediate and high groups, significantly differing from the low group (p<0.001). Table 1 presents maternal epidemiological and medical characteristics. Mothers in the intermediate and high sFlt-1/PlGF ratio groups were notably younger compared to those in the Low group (p<0.05).
| Parameter | Low (A) | Intermediate (B) | High (C) |
|---|---|---|---|
| A-indication for sFlt1/PlGF testing | |||
| Preeclampsia | 6 | 27 | 27 |
| Hypertension | 26 | 8 | 14 |
| HELLP syndrome | 0 | 0 | 2 |
| Suspicion of Preeclampsia | 23 | 16 | 8 |
| Thrombophilia | 0 | 0 | 1 |
| FGR | 5 | 9 | 8 |
| B-maternal medical history (Mean ± SD/N (%)) | |||
| Maternal age | 35,65 ± 5,59 | 33,17 ± 5,30 | 33,88 ± 5,81 |
| Parity | - | - | - |
| 0 | 25 (41.7) | 25 (41.7) | 29 (48.3) |
| 1 | 12 (20) | 19 (31.7) | 15 (25) |
| 2 | 10 (16.7) | 12 (20) | 10 (16.7) |
| ≥3 | 13 (21.7) | 4 (6.7) | 6 (10) |
| Smoking | 9 (15) | 4 (6.7) | 9 (15) |
| Ethnicity | |||
| Caucasic | 50 (83.3) | 45 (75) | 46 (76.7) |
| Latin | 7 (11.7) | 10 (16.7) | 13 (21.7) |
| Black | 2 (3.3) | 2 (3.3) | 0 (0) |
| North-African | 1 (1.7) | 3 (5) | 1 (1.7) |
| Assisted reproduction | |||
| None | - | - | - |
| IVF | 7 (11.7) | 9 (15) | 8 (13.3) |
| Ovodonation | 7 (11.7) | 1 (1.7) | 1 (1.7) |
| Diabetes | |||
| None | 53 | 51 | 52 |
| Pregestational | 7 (11.7) | 8 (13.3) | 4 (6.7) |
| Gestational | 0 (0) | 1 (1.7) | 4 (6.7) |
| Thrombophilia | 1 (1.7) | 2 (3.3) | 2 (3.3) |
| Lupus | 4 (6.7) | 0 (0) | 1 (1.7) |
| Kidney disease | 2 (3.3) | 1 (1.7) | 5 (8.3) |
| Previous FGR | 7 (11.7) | 4 (6.7) | 3 (5) |
| Obesity | 1 (1.7) | 4 (6.7) | 4 (6.7) |
| COVID in pregnancy | 1 (1.7) | 1 (1.7) | 1 (1.7) |
Note: HELLP syndrome: Hemolysis, Elevated Liver Enzyme Levels and Low Platelet Levels; FGR: Fetal Growth Restriction; PIGF: Placental Growth Factor; sFlt1: Soluble fms-like Tyrosine Kinase-1; IVF: In Vitro Fertilization; COVID: Coronavirus Disease; SD: Standard Deviation.
Table 1: Descriptive analysis of maternal characteristics.
Biophysical and biochemical parameters at study entry
The Table 2 displays the biophysical and biochemical parameters measured at the beginning of the study. A gradient of AMO severity-High>Intermediate>Low is evident for systolic blood pressure, mean blood pressure, angiogenic markers, AST, ALT and sFlt-1/PlGF ratio.
| Parameter | Low (A) | Intermediate (B) | High (C) |
|---|---|---|---|
| Maternal examination | |||
| Blood test (week) | 34,04 ± 4,12 | 32,31 ± 3,40 | 28,50 ± 3,47 |
| Systolic BP | 149,87 ± 17,43 | 153,18 ± 13,02 | 167,80 ± 27,45 |
| Dyastolic BP | 94,95 ± 16,14 | 98,38 ± 10,27 | 99,60 ±14,70 |
| MAP | 113,26 ± 15,01 | 116,65 ± 9,70 | 122,23 ± 17,64 |
| sFlt1/PlGF | 37,17 ± 23,81 | 237,10 ± 143,15 | 996,08 ± 414,04 |
| sFlt1 | 4813,22 ± 2785,36 | 11738,12 ± 6744,52 | 18881,28 ± 11566,23 |
| PlGF | 199,08 ± 168,15 | 58,26 ± 31,96 | 21,04 ± 14,94 |
| Uric acid | 6,81 ± 6,16 | 5,95 ± 1,57 | 6,05 ± 1,23 |
| AST | 17,39 ± 10,55 | 28,10 ± 16,81 | 59,62 ± 107,39 |
| ALT | 17,69 ± 17,96 | 31,08 ± 32,30 | 67,59 ± 151,69 |
| Platelets | 225,34 ± 57,81 | 211,18 ± 69,04 | 201,82 ± 75,85 |
Note: sFlt1: Soluble fms-like Tyrosine Kinase-1; PIGF: Placental Growth Factor; AST: Aspartate Amino Transferase; ALT: Alanine Transaminase; BP: Blood Pressure; MAP: Mean Arterial Pressure.
Table 2: Biophysical and biochemical parameters at study entry.
PlGF, sFlt-1 and the sFlt-1/PlGF ratio correlated with clinical and analytical variables, except for uric acid and platelets, as illustrated in Table 3.
| sFlt1/PlGF | sFlt1 | PlGF | |
|---|---|---|---|
| sFlt1/PlGF, N=180 | - | 0.78*** | -0.88*** |
| sFlt1, N=180 | 0.78*** | - | -0.39*** |
| PIGF, N=180 | -0.88*** | -0.39*** | - |
| Proteinuria, N=151 | 0.3*** | 0.32*** | -0.214*** |
| Uric acid, N=118 | -0.04, NS | -0.1, NS | -0.04, NS |
| AST, N=160 | 0.229** | 0.28** | -0.11, NS |
| ALT, N=162 | 0.190* | 0.26** | -0.07, NS |
| Platelets, N=163 | -0.13 (NS) | -0.21** | 0.02, NS |
| Systolic blood pressure, N=179 | 0.34*** | 0.18* | -0.37*** |
| Dyastolic blood pressure, N=179 | 0.18* | 0.120, NS | -0.17* |
| Mean arterial pressure, N=179 | 0.28*** | 0.160* | -0.28*** |
| Delivery week, N=180 | -0.71*** | -0.45*** | 0.71*** |
| Interval to delivery, N=180 | -0.53*** | -0.47*** | 0.43*** |
| Risk of Preeclampsia, N=180 | 0.28*** | 0.18* | -0.28*** |
Note: NS: Non-Significant; (*): p<0.05, (**): p<0.01, (***): p<0.001; sFlt1: Soluble fms-like Tyrosine Kinase-1; PIGF: Placental Growth Factor; AST: Aspartate Amino Transferase; ALT: Alanine Transaminase.
Table 3: Pearson correlation values between clinical variables and angiogenic factors.
Adverse Maternal Outcomes (AMO) in the Study
The adverse maternal outcomes observed in our study included severe preeclampsia, (Hemolysis, Elevated Liver Enzyme Levels and Low Platelet Levels) HELLP syndrome, abruption placentae and Intensive Care Unit (ICU) admission. We observed a graded potency of AMO severity across the High>Intermediate>Low groups (p<0.001 for each comparison). The Table 4 illustrates that the interval between study entry and delivery was inversely related to the level of the sFlt-1/PlGF ratio (p<0.001).
| Low (A) | Intermediate (B) | High (C) | |
|---|---|---|---|
| Interval exam-delivery (days) | 23,22 ± 26,88 | 13,00 ± 13,32 | 4,58 ± 8,02 |
| Normal outcome | 34 | 21 | 4 |
| Chronic hypertension | 6 | 2 | 0 |
| Gestational hypertension | 8 | 0 | 0 |
| Preeclampsia | 10 | 14 | 9 |
| Severe Preeclampsia* | 2 | 17 | 34 |
| HELLP syndrome* | 0 | 4 | 13 |
| Abruptio placentae* | 0 | 2 | 0 |
| ICU admission* | 2 | 10 | 18 |
Note: ICU: Intensive Care Unit; HELLP syndrome: Hemolysis, Elevated Liver Enzyme Levels and Low Platelet Levels; (*):The presence of any of these parameters defined the existence of adverse maternal outcome.
Table 4: This table representsmaternal outcome as subsequent maternal evolution.
The median sFlt-1/PlGF ratio and interquartile range (25th percentiles and 75th percentiles) were 739 (352.5-927) for samples associated with AMO and 71 (29-211) for those not associated with AMO (Mann-Whitney, p<0.001).
We evaluated the performance and predictive capability of various angiogenic, clinical and analytical markers for AMO. The sFlt-1/PlGF ratio exhibited the highest AUC value, outperforming all other parameters studied, which had significantly lower AUCs: PlGF, sFlt-1, AST, ALT, platelets, systolic blood pressure, Pulmonary Embolism (PE) risk, proteinuria, Mean Arterial Pressure (MAP), diastolic blood pressure and uric acid as shown in Table 5.
| AUC | 95% CI | *p-value | |
|---|---|---|---|
| sFlt1/PlGF | 0,88 | 0,82 to 0,92 | - |
| 1/PlGF | 0,82 | 0,76 to 0,88 | 0,01 |
| sFlt1 | 0,80 | 0,73 to 0,86 | 0,001 |
| AST | 0,69 | 0,58 to 0,80 | 0,01 |
| ALT | 0,64 | 0,53 to 0,75 | 0,001 |
| Preeclampsia risk | 0,64 | 0,57 to 0,71 | 0,0001 |
| Platelets | 0,61 | 0,49 to 0,73 | 0,001 |
| Systolic blood pressure | 0,63 | 0,54 to 0,71 | 0,0001 |
| Proteinuria | 0,60 | 0,49 to 0,71 | 0,0001 |
| Mean arterial pressure | 0,59 | 0,50 to 0,67 | 0,0001 |
| Diastolic blood pressure | 0,54 | 0,45 to 0,63 | 0,0001 |
| Uric acid | 0,51 | 0,40 to 0,63 | 0,0001 |
| Model 1 | 0,84 | 0,76 to 0,91 | NS |
| Model 2 | 0,82 | 0,73 to 0,90 | NS |
Note: sFlt1: Soluble fms-like Tyrosine Kinase-1; PIGF: Placental Growth Factor; AST: Aspartate Amino Transferase; ALT: Alanine Transaminase. Model 1: Preeclampsia risk+sFlt1/PlGF ratio+Platelets; Model 2: Preeclampsia risk+sFlt1/PlGF ratio+Uric acid+AST; *p-values represent the difference with the sFlt1/PlGF which is represents the control parameter; AUC: Area Under the Curve; NS: Non-Significant; CI: Confidence Interval.
Table 5: Receiver operating characteristic curves for prediction of adverse maternal outcome. The AUC and the 95% CI is given for each potential predictor.
Logistic regression was used to develop several multivariate models for predicting AMO. The sFlt-1/PlGF ratio alone did not significantly differ from the best multivariate models: Model 1 (PE risk, sFlt-1/PlGF, platelets) with an AUC of 0.84 (95% CI: 0.76-0.91) and Model 2 (PE risk, sFlt-1/PlGF, uric acid and AST) with an AUC of 0.82 (95% CI: 0.73-0.90) (Table 5 and Figure 1). Notably, PE risk and sFlt-1/PlGF were independent factors in both models identified through logistic regression.
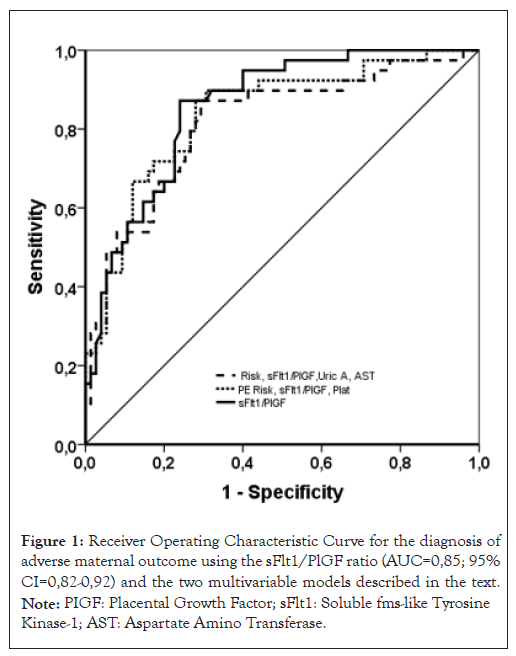
Figure 1: Receiver Operating Characteristic Curve for the diagnosis of adverse maternal outcome using the sFlt1/PlGF ratio (AUC=0,85; 95% CI=0,82-0,92) and the two multivariable models described in the text. Note: PIGF: Placental Growth Factor; sFlt1: Soluble fms-like Tyrosine Kinase-1; AST: Aspartate Amino Transferase.
To assess the diagnostic performance for AMO, we constructed a ROC curve for the sFlt-1/PlGF ratio, resulting in an AUC of 0.88 (95% CI: 0.82-0.92). The cut-off value with the highest accuracy, determined by Youden’s Index (J), was an sFlt-1/PlGF ratio of 137. At this threshold, the test demonstrated a sensitivity of 93.15% (95% CI: 84.7-97.7), a specificity of 70.09% (95% CI: 60.5-78.6), a positive Likelihood Ratio (+LR) of 3.11 (95% CI: 2.3-4.2) and a negative Likelihood Ratio (-LR) of 0.098 (95% CI: 0.04-0.2). Assuming a pre-test probability of 2%, the -LR of 0.098 and the +LR of 3.11 yielded a post-test probability of AMO of 0.2% for a negative test result and 7% for a positive test result as shown in Figure 2.
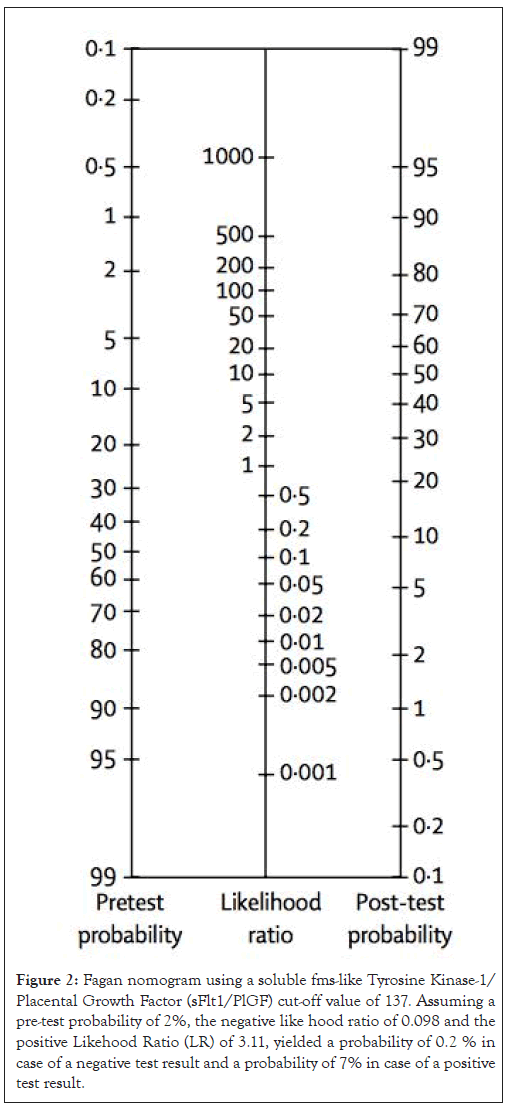
Figure 2: Fagan nomogram using a soluble fms-like Tyrosine Kinase-1/ Placental Growth Factor (sFlt1/PlGF) cut-off value of 137. Assuming a pre-test probability of 2%, the negative like hood ratio of 0.098 and the positive Likehood Ratio (LR) of 3.11, yielded a probability of 0.2 % in case of a negative test result and a probability of 7% in case of a positive test result.
Our study demonstrates that the sFlt-1/PlGF ratio is a valuable tool for estimating Adverse Maternal Outcomes (AMO). As an individual marker, the sFlt-1/PlGF ratio exhibited the highest diagnostic performance for predicting AMO, outperforming the two logistic regression models that included this ratio, highlighting its critical importance. The proposed cut-off point of 137 is particularly effective for ruling out AMO due to its very low negative likelihood ratio (0.098). However, it is less effective for ruling in AMO, with a positive likelihood ratio of 3.11.
Our findings are consistent with previously published data, indicating that a higher sFlt-1/PlGF ratio is associated with a shorter time to delivery and worse adverse maternal outcomes.
Differences in findings may stem from different methodological approaches, such as the gestational week considered (e.g., before 34 weeks, 35 weeks, 37 weeks, or throughout the entire pregnancy), the maternal complications studied (e.g., severe features of preeclampsia as defined by ACOG, FullPIERS or others), or the criteria used to determine preeclampsia (e.g., risk suspicion, confirmed diagnosis, or a combination of suspected and confirmed cases). These discrepancies have been highlighted by two systematic reviews.
Like our study, others have shown that the sFlt-1/PlGF ratio is a superior marker for Adverse Pregnancy Outcomes (APRO) compared to any isolated clinical or analytical parameter, as evidenced by its greater AUC. However, some authors have found that multivariate models, including sFlt-1/PlGF, clinical and other analytical markers, are better predictors of APRO, despite having similar AUC values to our study. The mentioned reviews have already pointed out the source of these differences. The consistency of these findings strongly suggests that angiogenic biomarkers will be useful for risk stratification in organizing settings.
Some authors have chosen a cut-off value of 85 for the sFlt-1/PlGF ratio to ascertain APRO. In women presenting at less than 34 weeks, a cut-off of 85 yielded a sensitivity of 72.9% and specificity of 94.0%. Our data, however, suggest a threshold of 137, with a sensitivity of 93.15%, specificity of 70.09%, positive LR of 3.11 and negative LR of 0.098. This negative LR, based on Bayesian Fagan estimations, would decrease the post-test probability about tenfold, to 0.2%, assuming an incidence of APRO of about 2%. Thus, the sFlt-1/PlGF cut-off of 137 is very effective at ruling out AMO but not for prognosis. This partially supports the NICE guidelines, which highlight the value of sFlt-1/PlGF in ruling out the presence of the disease. The differences between the cut-off values of 85 and 137 is due to our study covering the entire duration of pregnancy and focusing only on maternal outcomes, whereas the cut-off proposed by Rana, et al., [14]. Considers gestations less than 34 weeks and adverse maternal and perinatal outcomes.
The addition of the sFlt-1/PlGF ratio to the FullPIERS model has not been extensively studied. Only one study has examined this combination, finding that the sFlt-1/PlGF ratio correlated more closely with the number of adverse maternal outcomes than the PIERS model and was a superior predictor of maternal complications. However, the combined use of the sFlt-1/PlGF ratio and the PIERS model did not enhance the prediction accuracy for maternal complications.
Extremely high sFlt-1/PlGF ratio values were also reported by Leanos Miranda, et al., [23] in 2020: 610 ± 378 for severe preeclampsia and 764 ± 415 for HELLP syndrome and/or eclampsia, both significantly different from mild or severe gestational hypertension and mild preeclampsia. A controversial issue is whether a specific threshold can be set to strongly suspect or rule in adverse maternal outcomes. Very high sFlt-1/PlGF ratios (>655 for early-onset and >201 for late-onset preeclampsia) are believed to indicate a high risk of short-term complications and the need for delivery. Others have proposed a cut-off point of 178 for predicting complications such as imminent delivery or fetal/neonatal death. The Stolz, et al., [15] suggested that a sFlt-1/PlGF ratio above 1000 is more useful for predicting perinatal adverse outcomes associated with preeclampsia. Mirkovic, et al., [22] proposed a ratio of 377 for predicting adverse maternal outcomes (AUC 0.853, 95% CI 0.733-0.972). While our sFlt-1/PlGF AUC values are quite similar, the cut-off points we propose are different. These differences, despite both studies analysing maternal adverse outcomes, can be attributed to several factors: Analyzed outcomes similar to those in the Preeclampsia Integrated Estimate of Risk Study (PIERS), with clinical adverse outcomes required to manifest within seven days. However, they included patients with a diagnosis of early severe preeclampsia between 24 and 34 weeks, whereas we included cases of suspected and diagnosed placental insufficiency from 21 weeks to term.
Clinical implications
Despite consensus guidelines outlining indications for delivery in patients with preeclampsia, risk assessment remains challenging. This is because no single sign, symptom or laboratory test has yet been shown to predict adverse outcomes with high accuracy. Nevertheless, the strong association of the sFlt-1/PlGF ratio with severe maternal morbidity, combined with its predictive performance, makes it an important tool in assessing the risk of adverse maternal outcomes associated with suspected or established preeclampsia. Clinical markers should also be considered, as demonstrated in various studies. Due to differences in methodology, there is significant discrepancy in the proposed cut-off points for estimating adverse maternal outcomes. We identified 137 as an optimal cut-off point for ruling out adverse maternal outcomes. However, further studies are needed to establish a reliable cut-off for ruling in adverse maternal outcomes.
Research implications
Many sFlt-1/PlGF cut-off points have been proposed to diagnose preeclampsia as well as their complications. Therefore, it may be important to normalize the values by converting them to Multiples of the Median (MoM) and to standardize the outcomes, in order to develop more robust cut-off points.
As previously stated, differences in methodology make it difficult to establish stable values for estimating the risk of Adverse Outcomes (AO). In cases of placental insufficiency, maternal and fetal interests may diverge, so studies should analyze both separately and together to better estimate AO. The stratification of the population into three groups and the wide range of sFlt-1/PlGF values in the sample allowed us to more clearly identify the association of different maternal adverse outcomes as a function of the sFlt-1/PlGF ratio. Including women with suspected or confirmed placental dysfunction enabled us to mimic the real clinical setting where the sFlt-1/PlGF ratio will be used.
Other strengths include the consistent results with those obtained in the literature and the importance of including clinical manifestations or risk factors in models predicting AO. This is a single-centre study with a relatively small sample size. Due to the low prevalence of maternal AO manifestations, our study was not powered to fully assess all of them. The issue of low prevalence has also been noted by others. A potential bias in this study is that clinicians were not blinded to the sFlt-1/PlGF values; therefore, management could have been influenced by these results and by their experience with angiogenic biomarkers.
This study, like others in the literature, relies on absolute sFlt-1/PlGF ratio values. Since the distribution of these values across pregnancy is shifted, it might be more appropriate to express them in terms of multiples of the median for gestational age.
The sFlt-1/PlGF ratio has a dose dependent relation with the severity of the AMO and can be used in the clinical setting to predict the prognosis of preeclampsia. SFlt-1/PlGF ratio appears to be a better maker of AO than any isolated parameter either clinical or analytical. We propose the 137 as the new cut off for ruling out the AMO in patients with placental insufficiency. Significant differences were observed among the sFlt-1/PlGF groups (High>Intermediate>Low) for systolic and mean blood pressure, angiogenic markers, Aspartate Aminotransferase (AST), Alanine Transaminase (ALT) and AMO (p<0.001). The sFlt-1/PlGF ratio showed the highest Area Under the Curve (AUC) for predicting AMO compared to individual parameters were observed.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[PubMed]
[PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Martin AP, Cruz FF, Triguero MM, Redondo AA, Ortiz RM, Novillo-del-Alamo B, et al. (2024). The sFlt-1/PlGF Ratio as Predictor of Adverse Maternal Outcome in Patients with Suspected Placental Insufficiency. Clin Pediatr. 09:277.
Received: 23-Aug-2024, Manuscript No. CPOA-24-30808; Editor assigned: 26-Aug-2024, Pre QC No. CPOA-24-30808 (PQ); Reviewed: 09-Sep-2024, QC No. CPOA-24-30808; Revised: 16-Sep-2024, Manuscript No. CPOA-24-30808 (R); Published: 23-Sep-2024 , DOI: 10.35248/2572-0775.24.09.277
Copyright: © 2024 Martin AP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.