
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Research Article - (2022)Volume 8, Issue 4
Purpose: EGFR-TKIs are the first-line therapy for advanced NSCLC harboring EGFR-sensitive mutations. A robust immunity is an essential foundation for patients to tolerate continuous drug treatments. Lienal Polypeptide (LP) is an immunomodulator widely applied to regulate immunity in clinical practice. Nevertheless, its potential impact on EGFR-TKIs therapy has not been illustrated. This study aimed to explore the immunomodulatory and antitumor efficacy of LP in combination with EGFR-TKIs threapy in advanced NSCLC.
Patients and methods: Retrospective analysis on variation of lymphocytes in 106 NSCLC patients after EGFR-TKIs combined with LP treatment was performed. Proliferation experiment, transwell and wound healing assays were performed in PC9-GR cells to estimate influence of LP on tumor proliferation, invasion and migration in vitro. Flow cytometry was performed to detect cell apoptosis and cell cycle. The expression of p-EGFR and EGFR were detected by Western blot to investigate antitumor effect of LP.
Results: The levels of CD3+, CD4+, CD8+ T-cells and the CD4+/CD8+ ratio were higher in NSCLC patients treated with Gefitinib in conjunction with LP. Gefitinib combined with LP inhibited tumor prolifertaion, invasion and migration, as well as promoted cell apoptosis in vitro.
Conclusion: LP had a synergistic anticancer effect with EGFR-TKIs in advanced NSCLC. LP in combination with EGFR-TKIs therapy has clinical curative effect in treatment of advanced NSCLC with EGFR driving mutations, can effectively enhance physical immunity and desensitized drug-resistant cells to EGFR-TKIs, which has a certain clinical application value.
Lienal polypeptide; EGFR-TKIs; Drug resistance; Combination therapy
Lung cancer remains the leading cause of cancer related deaths worldwide [1]. Non-small cell lung cancer (NSCLC) is divided to different molecular subtypes, among which epidermal growth factor receptor (EGFR) mutation is the most common subtype [2-4]. EGFR-TKIs such as Gefitinib extraordinarily prolonged median overall survival (OS) of advanced NSCLC patients, as well as improved the quality of life [5]. EGFR-TKIs have been recommended as first-line treatment for patients with advanced NSCLC harboring EGFR mutations [6-8]. Nevertheless, a considerable proportion of patients have to discontinue treatment due to adverse effects, which lead to disease progression and failure of therapy [9,10]. Long-term inflammatory responses due to treatment cause massive infiltration of inflammatory cells and increased cytokines levels. Such tumor micro-environment flooding with chronic inflammatory cells and inflammatory mediators may result in gene silencing or abnormal expression, epigenetic changes, mismatched repair enzyme inactivation, DNA damage or gene mutation in tumor cells, eventually contributing to malignant transformation and drug resistance of tumor cells [11-14].
Lienal Polypeptide (LP) is extracted from the spleen of healthy calves, which functioning as an immune modulator with the ability of correcting immune dysfunction, activating non-specific immune function, as well as improving the immune function of lymphocytes, therefore enhancing the body's defensive capabilities to infection [15]. At present, LP is mainly applied to cellular immunodeficiency diseases and malignant tumors caused by chemo-radiotherapy, which can ameliorate cancer cachexia [16,17]. LP has also been widely used in the treatment of multiple malignancy tumors nowadays.
Recently studies found that EGFR-TKIs can moderate T-lymphocytes and natural killer cells to deregulate carcinogenesis [18]. However the chronic inflammatory reaction induced by EGFR-TKIs inversely results in treating termination or drug resistance. LP, an immunomodifier, could significantly improve immune function and correct immune disorders. Therefore, we hypothesized that LP treatment could reduce the persistent chronic inflammation and drug-related adverse effects caused by EGFR-TKIs in NSCLC. In this study, we aim to use Gefitinib combined with LP to treat NSCLC harboring EGFR-sensitive mutations, observe the efficacy of the combined therapy, and investigate the effects of drug combination on tumor biological behaviors and immune function, we further clarified the molecular mechanisms of combination therapy. In conclusion, our study provides new insights in LP combined with EGFR-TKIs in treatment of advanced NSCLC.
Patients
A total of 106 patients diagnosed with III and IV stage NSCLC in Jiangsu Province Hospital from January 2019 to January 2021 were enrolled in this study. This study was approved by the ethical committee of the Jiangsu Province Hospital Medical Ethics Committee and was carried out in accordance with the approved guidelines. All participants had a good knowledge about the study and signed written informed consents. The inclusion criteria were as follows: Pathologically diagnosed with lung adenocarcinoma, carrying EGFR mutation concluding in-frame deletions in exon 19 (19DEL) or a point mutation in exon 21 (L858R), accepting oral administration of EGFR-TKIs per day and/or combined with intravenous infusion of lienal polypeptide liquid injection once a month (a treatment cycle). Clinical characteristics were described in Table 1.
Cell culture and agents
The human NSCLC cell line PC9 and the Gefitinib resistant cell line PC9-GR were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI 1640 or DMEM (GIBCO-BRL) medium which was supplemented with 10% fetal bovine serum (10% FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified incubator at 37°C with 5% CO2. Lienal polypeptide injection (Batch No. 20130405) was given by Jilin Fengsheng Pharmaceutical Co., Ltd (Jilin, China). Gefitinib was purchased from AstraZeneca Biotechnology limited company (London, England, UK).
Cell grouping and treatment
PC9 and PC9-GR cells were cultured in different concentration gradients of LP and logarithmic phase growth cells were collected for further study. PC9-GR cells were respectively grouped into: Gefitinib group (treated with Gefitinib), LP group (treated with lienal popypeptide liquid), Gefitinib-LP group (treated with Gefitinib and lienal polypeptide liquid) and control group (treated with DMSO).
Cell proliferation experiment
Cell proliferation was measured by CCK8 assay (cell counting kit-8, Selleck, Shanghai, China), colony-forming assay and BeyoClick™ EdU Cell Proliferation Kit with Alexa Fluor 488 (#C0071S; Beyotime Biotechnology) according to the manufacturer's instructions. In CCK-8 assays, PC9 cells and PC9-GR cells under logarithmic phase were seeded in 96-well plates maintaining in media containing 10% FBS at a density of 3500 cells/well and incubated overnight. Subsequently, the cells were exposed to different concentrations of LP for 72 h. After that, 10 μL of CCK8 was added into each well and incubated at 37°C for 1 hour. The optical density was measured at 450 nm by an enzyme-labeled instrument.
In colony formation experiment, PC9-GR cells were placed into a six-well plate with the density of 1000 cells a well and cultured in the medium containing 10% FBS for 14 days, culturing medium was replaced every 4 days. Colonies were fixed with methanol and stained with 0.1% crystal violet (Sigma-Aldrich, St.Louis, MO, USA) in PBS for 15 min. Colony formation was detected by counting the number of stained colonies..
In EdU labeling assay, PC9-GR cells were incubated with 10 μM EdU solutions for 2 hrs after treatment with drugs for 48 h. Then cells were fixed and permeabilized and cultured in click additive solution for 30 min. Finally, the cell nucleus was stained with Hoechst 33342 solution for 10 minutes. The results were observed and captured using the fluorescence microscope (Olympus Corporation) and the percentage of positively stained cells was calculated as the proliferation rate.
Wound healing assay
On the back of the 6‐well plate, a marker pen was used to draw uniform horizontal lines with the assistance of a straightedge. The lines were at intervals of 0.8 cm and crossing the wells, with at least five lines for each well. In each well, 5 × 105 cells were added, and the confluency reached 100%. 24 hours later, the pipette (20 μL) was used to scratch along the straightedge vertical to the horizontal lines on the back. After scratching, cells were rinsed by PBS for three times to remove the scratched cells. With culture medium, the plate was incubated in a 5% CO2 incubator at 37°C. The samples were collected at 0, 24, 48 hours and photographed under an inverted microscope. The healing area of scratches was calculated by National Instrument Vision Assistant 8.6 software: migration rate=healing area of scratch/initial area of scratch × 100%. Experiments were carried out three times and mean value was calculated.
Transwell assay
Cells were digested after culturing in different groups. Every 5 × 104 cells in serum-free RPMI 1640 were seeded in the upper chamber (8 mm; Millipore), and the lower chamber was added with RPMI 1640 containing 10% FBS. After 24 hours’ incubation, the cells migrated through the membrane were fixed by 4% paraformaldehyde for 15 minutes and stained with 0.1% crystal violet for 10 minutes. The images were taken by an IX7 inverted microscope (Olympus, Tokyo, Japan), five fields of view were randomly selected for photographing and counting. The number of cells adhering to the Matrigel of the side in the lower chamber was considered as the number of invasive cells. All experiments were conducted in triplicate.
Flow cytometric analysis of apoptosis
After cultured for 48 hours described above in different groups, The PC9-GR cells were harvested by trypsinization and double stained with fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide using the FITC Annexin V apoptosis detection kit (BD Biosciences). Cell apoptosis ratio was determined by a flow cytometer (FACScan, BD Biosciences). Every experiment was performed three times independently.
Statistical analysis
SPSS 17.0 statistical software (Chicago, IL, USA) was used for the statistical analysis. The independent samples t-test was used to compare the changes of CD3+, CD4+, CD8+ T cells, NK cell activity and CD4+/CD8+ of peripheral blood between two groups of patients before and after therapy. P<0.05 was considered statistically significant.
Patients’ characteristics
A total of 106 patients carrying EGFR mutations (19DEL and 21L858R) and diagnosed with advanced NSCLC were enrolled in this study. 53 of them were only oral administered with EGFRTKIs, named control group, and remaining 53 participants received combined treatment of LP and EGFR-TKIs, named treatment group. The control group consisted of 27 males and 26 females, with a median age of 61.9 ± 8.2 years, and accordingly 28 males and 25 females in treatment group, with a median age of 62.3 ± 8.4 years. III and IV stage patients were 7 and 46 in control group, while 11 and 42 in treatment group. Detailed information including smoking history, EGFR mutation subtypes and the type of EGFR-TKIs were shown in Table 1.
| Characteristic | Control group | Treatment group |
|---|---|---|
| Sex, n (%) | ||
| Female | 26(49) | 25(47) |
| Male | 27(51) | 28(53) |
| Age, years(%) | ||
| <65 | 35(66) | 29(55) |
| ≥ 65 | 18(34) | 24(45) |
| Median age, years | 61.9 ± 8.2 | 62.3 ± 8.4 |
| Smoking history, (%) | ||
| Yes | 9(17) | 8(15) |
| No | 44 (83) | 45 (85) |
| TNM Stage, n (%) | ||
| III | 7(13) | 11(20) |
| IV | 46(87) | 42(80) |
| EGFR mutation, n (%) | ||
| 19DEL | 28(53) | 23(43) |
| 21L858R | 25(47) | 30(57) |
| EGFR-TKIs, n (%) | ||
| Gefitinib | 17(32) | 21(40) |
| Eerlotinib | 1(2) | 0(0) |
| Icotinib | 29(54) | 27(50) |
| Afatinib | 2(4) | 2(4) |
| Osimertinib | 4(8) | 1(2) |
| Almonertinib | 0(0) | 2(4) |
| Total, n (%) | 53(100) | 53(100) |
Table 1: Patient characteristics. Baseline characteristics and treatment information of 106 patients included in this study. The control group refers to patients who were orally administered EGFR-TKIs only. The treatment group included patients who received the treatment of EGFR-TKIs combined with lienal polypeptide injection.
LP combined with Gefitinib enhances immunity in advanced NSCLC
To investigate the immune regulating ability of LP in real world, we retrospectively analyzed the changes of lymphocyte populations involving CD3+ T cells, CD4+ T cells, CD8+ T cells, natural killer (NK) cells, B lymphocytes and the ratio of CD4+/CD8+ in peripheral blood before and after treatment (Figure 1 and Table 2). Compared with control group,the levels of CD3+, CD4+ and CD8+ T lymphcytes increased dramatically in the treatment group and had significant differences (p<0.05). In particular, the ratio of CD4+/ CD8+ displayed an upward trend in the treatment group after the related treatment. On the contrary, all the above indicators showed a descending tendency in control group (p<0.05). These results indicated that LP combined with Gefitinib presented a notable benefit in improving immune function in advanced NSCLC patients.
| Lymphocyte subtypes | Control group (n=53) | Treatment group (n=53) | ||
|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | |
| CD3+ (%) | 70.19 ± 1.47 | 59.74 ± 13.02a | 65.52 ± 11.07 | 74.21 ± 9.12 a,b |
| CD4+ (%) | 40.84 ± 10.89 | 32.33 ± 11.71 a | 36.85 ± 9.68 | 42.31 ± 10.50 a,b |
| CD8+ (%) | 24.34 ± 10.06 | 23.24 ± 9.37 | 25.71 ± 8.56 | 27.87 ± 10.43 a,b |
| NK (%) | 17.20 ± 10.98 | 27.73 ± 15.32 a | 18.47 ± 11.34 | 13.58 ± 8.19 a,b |
| B (%) | 10.60 ± 4.71 | 10.03 ± 6.66 | 10.71 ± 4.82 | 10.08 ± 4.51 a |
| The ratio of CD4+/CD8+ | 2.08 ± 1.31 | 1.83 ± 1.18 a | 1.65 ± 0.89 | 1.81 ± 0.95 a,b |
Note: a: p<0.05 compared with treatments; b: p<0.05 compared with control group.
Table 2: Changes in peripheral blood lymphocyte subtypes of 106 NSCLC patients after different treatments in two groups.
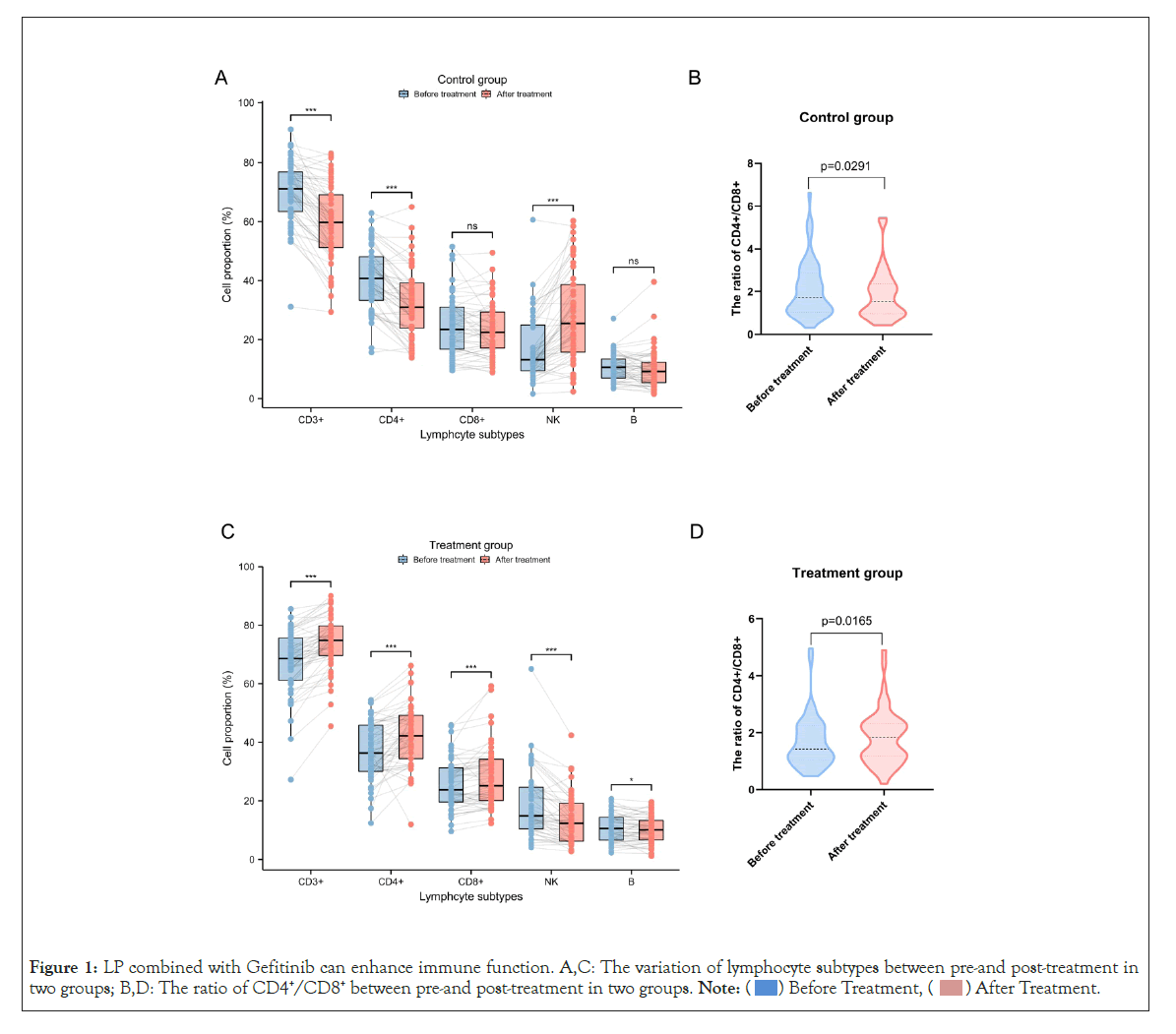
Figure 1: LP combined with Gefitinib can enhance immune function. A,C: The variation of lymphocyte subtypes between pre-and post-treatment in
two groups; B,D: The ratio of CD4+/CD8+ between pre-and post-treatment in two groups.
LP combined with Gefitinib represses tumor growth of PC9-GR cells
As in previous study demonstrated that EGFR-TKIs resistant tumors possess an immunosupressive microenvironment with relative more immunosuppressive cells and fewer immune-activated cells [18]. We hypothesise exogenous supplementation of immunomodulator could regulate intrinsic characteristics of tumor microenviroment, so as to increase sensitivity to EGFR-TKIs. Therefore, we investigate the influence of LP on Gefitinib-resistant cells PC9-GR in vitro. As shown in Figure 2A and 2B, LP exhibited an encouraging effect on inhibiting PC9-GR cell proliferation and this effect exhibited a dose-dependent manner. Compared with Gefitinib group, cell proliferation in Gefitnib-LP group was significantly inhibited (p<0.05), with IC50 values of 2.405 mg/ml in Gefitinib group versus 1.653 mg/ml in Gefitinib-LP group. Furthermore, EdU labeling assay (Figure 2C) and cloning formation experiments (Figure 2D) showed the same result that cell proliferation was particularly diminished in Gefitinib-LP group. Collectively, these data indicated that LP could enhance sensitivity of PC9-GR to Gefitinib by repressing cell proliferation.
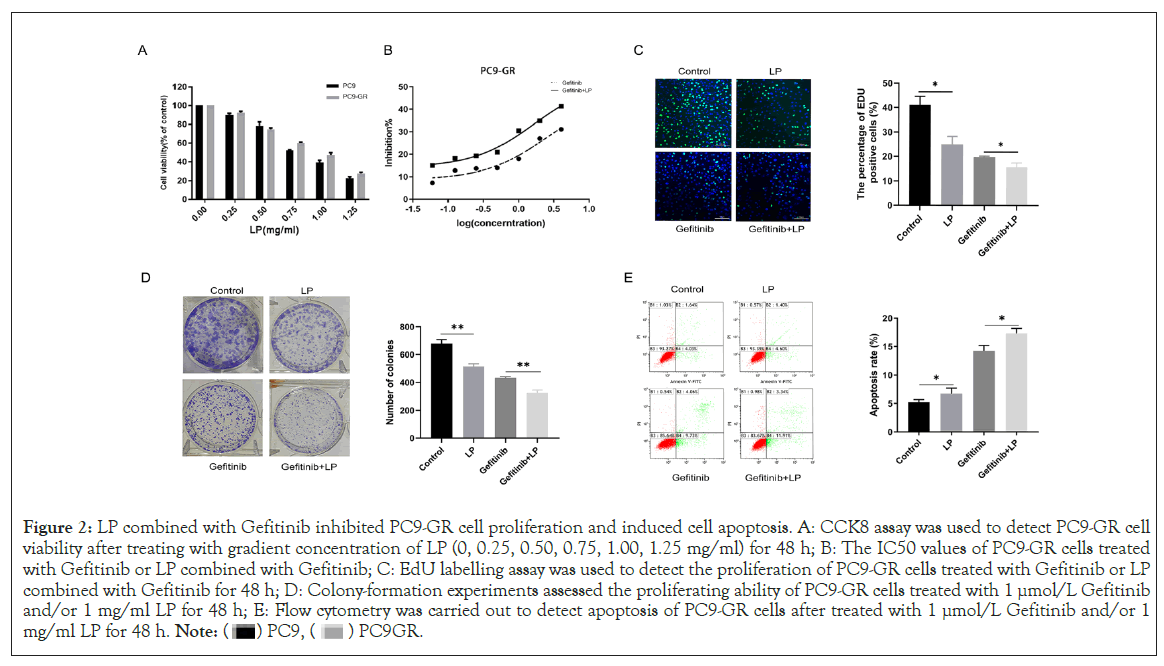
Figure 2: LP combined with Gefitinib inhibited PC9-GR cell proliferation and induced cell apoptosis. A: CCK8 assay was used to detect PC9-GR cell
viability after treating with gradient concentration of LP (0, 0.25, 0.50, 0.75, 1.00, 1.25 mg/ml) for 48 h; B: The IC50 values of PC9-GR cells treated
with Gefitinib or LP combined with Gefitinib; C: EdU labelling assay was used to detect the proliferation of PC9-GR cells treated with Gefitinib or LP
combined with Gefitinib for 48 h; D: Colony-formation experiments assessed the proliferating ability of PC9-GR cells treated with 1 µmol/L Gefitinib
and/or 1 mg/ml LP for 48 h; E: Flow cytometry was carried out to detect apoptosis of PC9-GR cells after treated with 1 µmol/L Gefitinib and/or 1
mg/ml LP for 48 h.
LP combined with Gefitinib promotes PC9-GR cell apoptosis in vitro
Cell apoptosis was detected by flow cytometry. As showed in Figure 2E, PC9-GR cells in Gefitinib-LP group had a higher apoptotic proportion than Gefitinib group (6.00% vs. 5.67%), namely, LP induced more cells to develop apoptosis, and the same phenomenon was observed in the Gefitinib-LP group versus Gefitinib group (15.25% versus 13.97%). These results demonstrated that LP combined with Gefitinib promoted PC9GR cell apoptosis.
LP combined with Gefitinib inhibits PC9-GR cell invasion and migration
Distant organ metastasis can generally be observed when EGFRTKIs therapy fails, subsequently resulting in recurrence or disease progression. To evaluate the function of LP on tumor metastasis, Wound healing experiment was used to estimate PC9-GR cell migration. Gefitinib-LP group showed a decrease in PC9-GR cell migration compared with other groups. The combination of Gefitinib with LP could markedly inhibit PC-9GR cell migration (Figure 3A). Transwell experiment was used to observe PC9-GR cell invasion ability. Compared with the control group, Gefitinib group and LP group, the number of invasive PC9-GR cells was reduced in Gefitinib-LP group (Figure 3B). These results suggested that LP might restore the sensitivity of PC9-GR cells to Gefitinib, hence further inhibiting tumor invasion and migration.
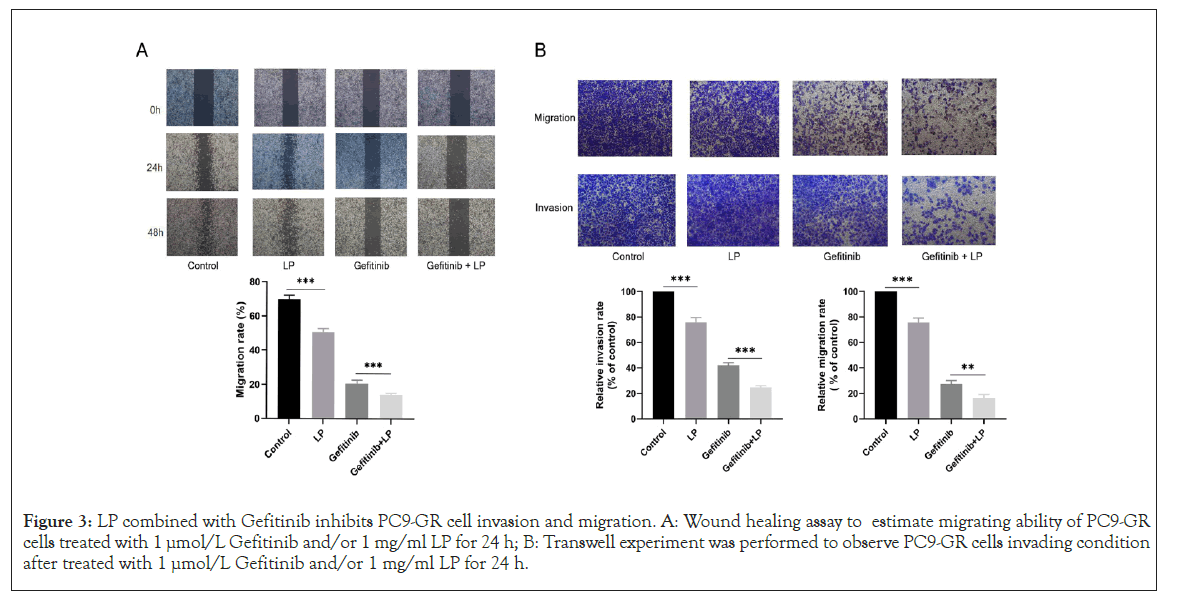
Figure 3: LP combined with Gefitinib inhibits PC9-GR cell invasion and migration. A: Wound healing assay to estimate migrating ability of PC9-GR cells treated with 1 µmol/L Gefitinib and/or 1 mg/ml LP for 24 h; B: Transwell experiment was performed to observe PC9-GR cells invading condition after treated with 1 µmol/L Gefitinib and/or 1 mg/ml LP for 24 h.
EGFR-TKIs play a dedicated therapeutic effect on NSCLC carrying EGFR sensitive mutations, such as EGFR 19 exon inframe deletion(19DEL) and substitutional mutation of arginine for leucine (L858R) in exon 21 [2,4,19]. Although EGFR-TKIs exhibited spectacular therapeutic benefits in clinical practice, the occurrence of adverse effects cannot be ignored at any time. Adverse events may impact the efficacy of anticancer therapies even result in treatment termination and disease progression. In addition to the reduction or stabilization of local lesions, systemic or local inflammatory reactions also occur in patients during the whole process of EGFR-TKIs therapy [18]. Common inflammatory reactions including rash, paronychia and chondriasis, hair disorders, mucitis, etc. Other inflammatory reactions include interstitial pneumonitis, chronic inflammation of intestinal tract and liver [20-22].
As an immune modulator, LP is generally prescribed in the treatment of numerous malignant tumors. Previous studies pointed out that LP can regulate the body’s immune function so as to improve physical condition of cancer patients and diminish cancer/ treatment-related painfulness or adverse effects [15]. Additionally, LP administration could elevate the cellular level of CD3+, CD4+ and NK cells in malignant cancer patients who underwent radiotherapies, which proved that LP could enhance the body's cellular immunity and reduce the toxicities of radiotherapy [16]. Similarly, our study showed higher levels of CD3+, CD4+ and CD8+ T lymphcytes and the ratio of CD4+/CD8+ in advanced NSCLC patients treated by LP combined with Gefitinib. CD3+ T cell is representative of whole immune cells level, normally the ratio of CD4+/CD8+ is larger than 1, the bigger the ratio is, the greater number of helper T cells are, and corresponding the less suppressor T cells are, which reflects a better immune status. Furthermore, increased CD4+/CD8+ ratio can serve as an independent prognostic factor in NSCLC [23-26]. Most importantly, CD8+ T lymphcytes play a central anticancer immunity. The enrichment of CD8+ T lymphcytes in tumor microenvironment on the one hand may help improve anticancer therapy, on the other hand could diminish the adverse effects duiring the related therapy so as to ensuring patients tolerating longer periods of treatment.
Our study innovatively found that LP showed a synergistic antitumor effect and could enhance the efficacy of EGFR-TKIs. LP combined with Gefitinib exhibited a more magnificent inhibitory capability on tumor cell biological behavior. A study focused on the combination therapy of cyclophosphamide (CTX) with LP based on murine lung carcinoma model pointed out that LP itself did not possess the property of direct antitumor effect, but it showed a synergistic enhancing phenomenon of antitumor effect when applied with CTX [27]. This probably attribute to LP relieving immunosuppressive tumor microenvironment by stimulating and activating lymphocytes in tumor microenvironment.
Taken together, our study demonstrated that LP in conjunction with Gefitinib could enhance body immune function so that empower patients tolerate longer treatment exposure and derive more benefit from EGFR-TKIs therapy, accordingly, arriving longer survival time. Furthermore, LP promotes the sensitivity of PC9- GR cells to EGFR-TKIs, inhibits tumor proliferation, invasion and migration, and consequencely enhances anticancer effect of EGFRTKIs therapy. In conclusion, LP in combination with Gefitinib was an effective treatment for patients of advanced NSCLC, which may improve the life quality of patients, and potentially improve prognosis. Further investigations are still required to explore the specific mechanisms to enhance the immunity of LP.
The work of this study was supported by grants from the National Natural Science Foundation of China (grant no. 81972188) and the Wu Jie-ping Foundation (320.6799.15032).
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
[Cross ref] [Google Scholar] [Pubmed].
Citation: Chen Y, Liu X, Yao J, Jin S, Li J, Xu J, et al. (2022) The Synergistic Antitumor Efficacy of Lienal Polypeptide Combined with EGFR-TKIs for Advanced NSCLC. Immunotherapy. 8:197.
Received: 04-Jul-2022, Manuscript No. IMT-22-18143 ; Editor assigned: 07-Jul-2022, Pre QC No. IMT-22-18143 (PQ); Reviewed: 22-Jul-2022, QC No. IMT-22-18143 ; Revised: 28-Jul-2022, Manuscript No. IMT-22-18143 (R); Published: 05-Aug-2022 , DOI: 10.35248/2471-9552.22.8.197
Copyright: © 2022 Chen Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.