
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research - (2022)Volume 5, Issue 3
Studies on immune-mediated neurological dysfunction over the last two decades have led to the development of number novel diagnostic and therapeutic opportunities. Neural autoantibodies can largely be classified based either on the localization of the antigen (synaptic/neuronal cell surface verses intracellular) or by etiology (autoimmune versus paraneoplastic). Neural autoantibodies can be detected in the blood and CSF and have the potential to serve as disease markers alone. This review summarizes the current understanding of pathophysiology of neural antigens recently identified in the last 15 years involved in paraneoplastic, idiopathic, and para-infectious disorders for which antibody testing is commercially available in the United States.
Autoimmunity; Encephalitis; Diagnostics; Neurology; Neuroimmunology
Over the last two decades, research in autoimmune diseases has led to a greater understanding of the neuronal pathophysiology of synaptic transmission and plasticity [1]. Autoimmune neurology is a rapidly developing field driven by the discovery of new neuronal antibodies associated with recognizable neurological syndromes (Figure 1). Neural autoantibodies are markers of an autoimmune or paraneoplastic origin of neurological symptoms; they can largely be classified two ways, firstly as two etiological categories of autoimmune (idiopathic, para-infectious) verse paraneoplastic (underlying cancer) or secondly as two antigenic categories including antibodies to synaptic/neuronal cell surface antigens verse antibodies to intracellular antigens. Deeper insights into the complex nature of various neurological disorders stemming from autoimmune responses to cell surface, intracellular or synaptic proteins have provided improved diagnostics and potential treatments (Table 1). Antibodies to intracellular antigens serve to identify various neurological disease markers and are normally inaccessible in intact cells. These autoantibodies can be detected in blood and CSF, are generally observed to be non-pathogenic, rather serve as disease biomarkers. Antigens found on the cell surface also serve as diagnostic biomarkers of neurological disorders and are typically considered to be pathogenic [33]. The focus of this review is to present the current understanding of pathophysiology of neural antigens recently identified in the last 15 years involved in paraneoplastic, idiopathic, and para-infectious disorders for which antibody testing is commercially available in the United States.
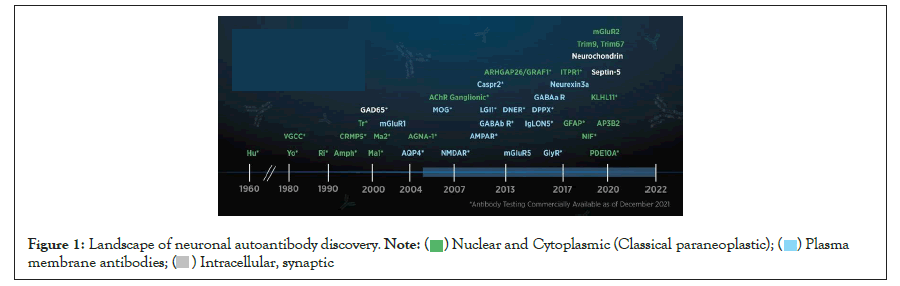
Figure 1: Landscape of neuronal autoantibody discovery.
| Antigen | Predominant Clinical Syndrome | Diagnostic Findings | Association with Cancer | Ref | |||
|---|---|---|---|---|---|---|---|
| Extracellular | |||||||
| NMDA receptor |
NMDA Receptor Encephalitis | CSF-IgG antibodies against the GluN1 subunit, pleocytosis or elevated IgG index, and normal or mild increase in proteins. | Ovarian teratoma, carcinomas | 1,2 | |||
| MRI-Normal or transient non-region-specific changes | |||||||
| AMPA receptor |
Limbic encephalitis | CSF-lymphocytic pleocytosis, and elevated protein concentration | Lung, Breast, Thymoma | 3,4 | |||
| MRI-Hyperintense signal highly restricted to medial temporal lobes | |||||||
| GABAb receptor |
Limbic encephalitis | CSF-lymphocytic pleocytosis, elevated protein concentration. | Lung, Neuroendocrine | 5,6 | |||
| MRI-Hyperintense signal highly restricted to medial temporal lobes | |||||||
| GABAa receptor | Encephalitis with prominent seizures | CSF-variable and abnormal in most cases and include pleocytosis, elevated protein concentration, or oligoclonal bands. | Infrequent | 1,7,8 | |||
| MRI-Hyperintense signal in multiple cortical and subcortical areas | |||||||
| LgI1 | Limbic encephalitis, Faciobracial dystonic seizures | CSF- lymphocytic pleocytosis (rare), and elevated protein concentration | Rarely thymoma (5-10% of cases) | 09-Dec | |||
| MRI-Hyperintense signal highly restricted to medial temporal lobes | |||||||
| CASPR2 | Encephalitis, Cerebellar ataxia, Morvan Syndrome, neuromyotonia | CSF- About 25% with abnormalities including pleocytosis and/or elevated protein, rarely OCB | SCLC, Thymoma | 13 | |||
| MRI-About half abnormal with T2 hyperintensities in temporal lobes or hippocampal/mesial temporal atrophy. | |||||||
| DPPX | Encephalitis with nervous system and GI hyper-excitability | CSF-pleocytosis, oligoclonal bands. | Lymphoma | 12,14 | |||
| MRI- Normal or non-region-specific changes | |||||||
| IgLON5 | Encephalitis with progressive sleep disorder and movement disorders | CSF-pleocytosis (rare), elevated protein. | No malignancies identified | 12,15 | |||
| MRI- normal | |||||||
| GlyR | Stiff-person syndrome (SPS), | CSF-Protein elevation, pleocytosis, and CSF-exclusive oligoclonal bands. Brain MRI often normal. | Thymomas, B-cell lymphoma, Hodgkin lymphoma, breast cancer, and small cell lung cancer | 16-18 | |||
| Progressive encephalomyelitis with rigidity and myoclonus (PERM) | |||||||
| AChR Ganglionic | Autoimmune dysautonomia | CSF-pleocytosis, increased protein levels and oligoclonal bands | Adenocarcinomas, small-cell lung cancer | 19 | |||
| MRI-normal or findings compatible with a diagnosis of multiple sclerosis. | |||||||
| MOG | Acute CNS demyelination | CSF-Neutrophilic pleocytosis WCC elevated >50% of the time with neutrophils presents in >70% of samples. CSF OCBs often negative. | Uncommon | 20 | |||
| MRI-Variable, longitudinally extensive spinal cord lesion, longitudinally extensive spinal cord atrophy, conus medullaris lesions. | |||||||
| Intracellular | |||||||
| Neurochondrin | Cerebellar ataxia | CSF-all with pleocytosis or OCB | No malignancies identified | 13 | |||
| MRI-Cerebellar and supratentorial gray matter atrophy | |||||||
| Homer-3 | Encephalitis, Cerebellar ataxia | CSF-pleocytosis, elevated IgG index in one patient | 1 SCLC identified | 13 | |||
| MRI-Variable, normal or cerebellar atrophy | |||||||
| ITPR1 | Cerebellar ataxia | CSF-pleocytosis and/or elevated protein | Breast, RCC, endometrial | 13,21,22 | |||
| MRI-mostly abnormal with cerebellar atrophy or midbrain/cerebellar T2 hyperintensities | |||||||
| KLHL11 | Encephalitis | CSF: Inflammatory in 85%; pleocytosis and protein elevation. Oligoclonal bands were detected in 82%. | Testicular germ-cell tumor | 23 | |||
| MRI: 76% had T2/FLAIR hyperintensity, three patients had gadolinium enhancement on MRI. | |||||||
| NIF | Cerebellar ataxia encephalopathy or myelopathy | CSF-inflammatory CSF (elevated lymphocyte-predominant white cell counts or CSF-restricted oligoclonal bands) | Neuroendocrine lineage | 24 | |||
| MRI-normal or cerebellar atrophy in ataxic patients, bilateral hippocampal T2 signal abnormalities in a patient with limbic encephalitis, and cranial nerve enhancement in 2 patients with cranial neuropathies. | |||||||
| GFAP | Meningoencephalomyelitis | CSF-high numbers of white blood cells including lymphocytes, monocytes, and multinucleate cells. Protein elevation. | Ovarian teratoma (particularly with coexisting NMDA or AQP4 abs) | 25-27 | |||
| MRI-Lesions involved the subcortical white matter, basal ganglia, hypothalamus, brainstem, cerebellum, meninges, ventricle, and skull. T2 hyperintensities and had gadolinium enhancement. | |||||||
| ARHGAP26 | Cerebellar ataxia | CSF-lymphocytic pleocytosis, elevated IgG index, and/or positive oligoclonal bands | Ovarian Carcinoma in 1 patient | 28-30 | |||
| MRI-atrophy of the cerebellar hemispheres and the cerebellar vermis | |||||||
| DNER (Tr) | Cerebellar ataxia | CSF-oligoclonal bands in ~60% of patients, pleocytosis and/or elevated protein | Hodgkin's lymphoma (high risk) | 1,31,32 | |||
| MRI- Normal or cerebellar atrophy | |||||||
NOTE: AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid); AQP4(Aquaporin-5); CSF(Cerebro Spinal Fluid); CASPR2, (Contactin-Associated Protein-2); DNER (Delta/Notch-like Epidermal growth factor-related Receptor) ; DPPX(Dipeptidyl-Peptidase-like Protein-6) ; GABAa (Gamma-Amino Butyric Acid type a); GABAb (Gamma-Amino Butyric Acid type b) ; ITPR1 (Inositol 1,4,5 Triphosphate Receptor type 1); LGI1 (Leucine-rich Glioma Inactivated 1); MRI(Magnetic Resonance Imaging); NMDA (N-Methyl-d-Aspartate); RCC(Renal Cell Cancer); SCLC (Small Cell Lung Cancer).
Table 1: Summary table of autoantibodies against neuronal targets.
Neural Surface Associated Proteins: Biomarkers in Autoimmune Encephalitis and Beyond
In contrast to most autoantibodies associated with paraneoplastic neurological symptoms, which often target intracellular antigens, most newly described autoantibodies in autoimmune encephalitis (AE) are directed against neuronal cell surface and synaptic antigens [1,33,34]. AE is a debilitating neurological disorder characterized by brain inflammation that leads to rapidly progressing encephalopathy. AE manifests with seizures and other neuropsychiatric symptoms. Proposed diagnostic criteria include possible, probable or definite AE based on clinical features and diagnostic tests including MRI features and CSF evaluation while carefully ruling out other disease that mimic AE [35]. Detection of antibodies against neuronal surface antigens identified in the cerebrospinal fluid and/or blood can lead to a more definitive diagnosis and is crucial, as often these syndromes are much more readily treatable with immunotherapy [34,35]. Such neuronal surface auto antigens will be described in detail below.
NMDA receptors: Anti-N-methyl-D-aspartate receptors (NMDA-R) are responsible for excitatory transmission mediated by α-amino- 3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) and the underlying process of synaptic plasticity. In NMDA-R encephalitis the target antigen of autoantibodies is the GluN1 of the NMDA receptor. Since its discovery in 2007, many cases of anti- NMDAR-associated AE have been reported, with an incidence of one in 1.5 per million people per year [36]. Anti-NMDA encephalitis is the most common neuronal surface antibody syndrome (NSAS) associated with AE, accounting for 80.95% of encephalitis cases in one study [37]. NMDA-R antibodies are associated with a characteristic syndrome that resembles a prodromal viral illness followed by psychiatric symptoms, catatonia, agitation, seizures, decreased level of consciousness, abnormal movements, and autonomic instability [33]. Anti-NMDA-R encephalitis is highly associated with ovarian teratomas [38]. Tumor removal in conjunction with immunotherapy is essential in the treatment course [39]. In patients with anti-NMDA-R encephalitis, CSF will show IgG antibodies against the GluN1 subunit in addition to inflammatory changes (i.e. pleocytosis and/or oligoclonal bands) [1,35]. The sensitivity for anti-NMDA autoantibody detection is higher in CSF, thus CSF evaluation is essential in the diagnostic work up [40]. As neuronal cell-surface antigens such as NMDA receptors are conformation dependent and fragile, solid phase detection methods such as Enzyme-Linked Immunosorbent Assay (ELISA) or immunoblot are not suitable. The optimal method for the detection of autoantibodies against NMDA and other cell- surface antigens is indirect immunofluorescence (IFA) using recombinant cells which can be complemented by neuronal tissue sections, and cultured primary neuronal cells [41]. A positive reaction of anti-NMDA receptor autoantibodies on neuronal tissue and cultured cells gives characteristic staining patterns.
AMPA receptors: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) is responsible for neural communication by causing depolarization at the postsynaptic membrane which results in rapid synaptic signaling, mediating precise transfer of information. Composed of homomeric or heteromeric subunits (GluA1-4) [42]. AMPA receptors mediate synaptic signaling based on the composition of these four core subunits. Antibodies to AMPA receptors target extracellular epitopes of glutamate receptor type 1 or type 2 subunits which cause receptor cross-linking and internalization and results in a reversible decrease in AMPA receptor clusters at synapses [3]. Anti-AMPA-receptor encephalitis has been described mostly in middle-aged women (90% women, range 38– 87 years) [3]. Anti-AMPA receptor encephalitis typically manifests as limbic encephalitis and is paraneoplastic in 64% of cases with tumors affecting the lung, breast, or thymoma. When anti-AMPA autoantibodies are found with antibodies against onconeuronal targets (e.g. CRMP5, amphiphysin) or tumor biomarkers linked to paraneoplastic autoimmunity (e.g., SRY-Box Transcription Factor 1 [SOX1]), the result is additional paraneoplastic symptoms and a poorer prognosis [4]. Comprehensive antibody testing for identification of antibodies in AE is important because their detection may inform prognostication.
CASPR2: Autoantibodies attributed to voltage-gated potassium channels (VGKC) were first described in 1995. Studies have since identified the clinically relevant antigens as contactin- associated protein-like 2 (CASPR2), a subgroup of the neurexin family consisting of 5 different proteins (CASPR1-CASPR5) thought to involved in the formation of excitatory or inhibitory synapses and leucine-rich, glioma inactivated 1 (LGI1) (described below) [43]. Anti-CASPR2 autoantibodies are associated with encephalitis and/or peripheral nerve hyperexcitability (known as Issaac’s syndrome or Morvan’s syndrome if there is peripheral and central nervous system involvement) [44]. A common symptom associated with anti-CASPR2 encephalitis is neuropathic pain (40%) [11]. Thymoma and other tumors in CASPR2 encephalitis has been reported to occur in 10-40% of cases [45]. It has been shown that high CASPR2 serum antibody titers indicate anti- CASPR2 encephalitis, a diagnostic sensitivity of 85% and a specificity of 81% [44]. Accuracy increases further when identified in combination with MRI findings [44]. The identification of anti- CASPR2 antibodies is important, as immunotherapy represents a successful treatment option.
LGI1: The LGI family consists of four conserved members (LGI1- LGI4) with widespread distribution in the observed in the brain [10]. The presence of LGI1 antibodies is associated with seizures (with faciobrachial dystonic seizures being pathognomonic) and encephalitis [10]. LGI1 is the main autoantigenic target of limbic encephalitis previously attributed to VGKC antibody-associated limbic encephalitis. LGI1-encephalitis affects men more often than woman (3:1) and 82% of patients with LGI1 antibodies have seizures [9]. Faciobrachial dystonic seizures may precede encephalitis; therefore, their recognition is important for early diagnosis and treatment [46]. Other features of LGI1 encephalitis include hyponatremia in 60% and abnormal EEGs in 76% of cases, with or without seizures [9]. The identification of serum LGI1 antibodies is a sensitive detection method. In one study that measured levels of serum anti-LGI1 antibodies were measured using an ELISA on a cell-based assay (CBA), 78% of patients who were positive for anti-LGI1 in serum, were also CSF positive [47].
DPPX: Dipeptidyl-peptidase-like protein-6 (DPPX) is a membrane bound glycoprotein. DPPX is a cell-surface voltage gated subunit of potassium channels (Kv4.2) with robust expression in the hippocampus, cerebellum and myenteric plexus. The largest case series to date includes 39 cases of anti-DPPX encephalitis [48]. Since this time, a few additional case reports have been published [49- 56]. Anti-DPPX encephalitis is characterized by central nervous system (CNS) hyperexcitability cognitive dysfunction, memory deficits, hyperekplexia, myoclonus, tremor, and seizures [57]. Gastrointestinal symptoms are also common and CNS symptoms are often preceded by unexplained weight loss with or without diarrhea. Criteria for the presence of DPPX antibodies included brain tissue immunostaining and CBA with human embryonic kidney 293 cells transfected with DPPX, as reported. One study found that antibodies are predominantly IgG1 and IgG4 and that their pathogenic effects on DPPX in cultured neurons are reversible on removal of the antibodies from the media [48]. Patients respond to immunotherapy regardless of the duration of symptoms prior to therapy which suggests that early diagnosis and treatment may further improve outcome [48].
GABA receptors: Gamma-Amino Butyric Acid (GABA) is the predominant inhibitory neurotransmitter in the CNS. GABA receptors are classified into three major groups (alpha, beta and gamma) and several minor ones. Ionotropic GABAa receptors are large-conductance voltage and calcium activated potassium channels that are responsible for both tonic and transient peri- and extra-synaptic responses while GABAb are metabotropic receptors positioned on postsynaptic axons and presynaptic terminals involved in regulation of excessive neurotransmitter release. Autoantibodies to the GABAa receptor are found patients with AE characterized by encephalitis, prominent seizures or status epilepticus, and multifocal MRI abnormalities (Figure 2) [1]. In patients with anti-GABAa antibodies, CSF findings are variable and abnormal in most cases and include pleocytosis, elevated protein concentration, or oligoconal bands. Roughly 30% of patients with Anti-GABAa AE present with a tumor such as a thymoma [1]. Anti-GABAa antibodies can also occur along with antibodies to glutamic acid decarboxylase 65 (GAD65), and GABAb receptor. The clinical syndrome associated with anti-GABAb antibodies is limbic encephalitis. This is characterized by memory dysfunction, behavioral effects, and seizures.5 GABAb encephalitis affects men and women equally and is commonly associated with small-cell lung cancer (SCLC) (in over 50% of patients) [5]. Autoimmunity to GABAb (and also AMPAR) may occur along with antibodies against intracellular antigens, such as thyroid peroxidase (TPO), GAD65, SOX1, and antinuclear antibodies, and also with antibodies against cell surface antigens such as N-type voltage-gated calcium channels [5,9].
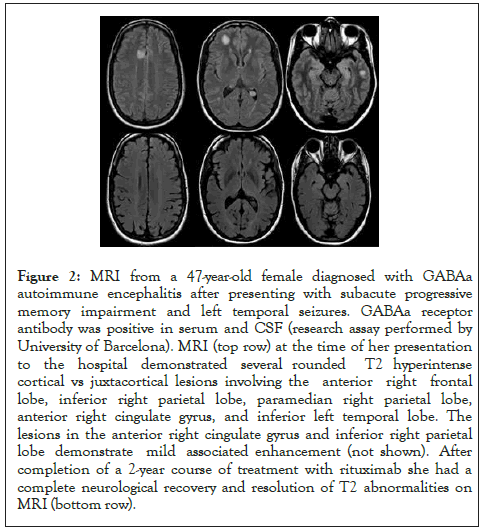
Figure 2: MRI from a 47-year-old female diagnosed with GABAa autoimmune encephalitis after presenting with subacute progressive memory impairment and left temporal seizures. GABAa receptor antibody was positive in serum and CSF (research assay performed by University of Barcelona). MRI (top row) at the time of her presentation to the hospital demonstrated several rounded T2 hyperintense cortical vs juxtacortical lesions involving the anterior right frontal lobe, inferior right parietal lobe, paramedian right parietal lobe, anterior right cingulate gyrus, and inferior left temporal lobe. The lesions in the anterior right cingulate gyrus and inferior right parietal lobe demonstrate mild associated enhancement (not shown). After completion of a 2-year course of treatment with rituximab she had a complete neurological recovery and resolution of T2 abnormalities on MRI (bottom row).
IgLON5: IgLON5 is an immunoglobulin-like cell adhesion molecule whose exact function is not well understood. IgLON5 autoimmunity is characterized as a progressive CNS disorder of insidious onset with prominent sleep and movement abnormalities. Symptoms include sleep-disordered breathing, gait instability, and various neuropsychiatric disorders. Progressive respiratory failure that leads to death is common. Indirect IFA screening can help identify anti-IgLON5 antibodies due to a unique staining pattern of diffuse neural synaptic (neuropil) staining [58]. In patients with IgLON5 autoimmunity, typical signs of autoimmunity such as subacute presentation, history of autoimmune disease, an inflammatory CSF, or inflammatory-appearing brain imaging are not commonly found. For this reason, antibody testing for IgLON5- IgG is important and it is recommended to test both serum and CSF for the presence of anti-IgLON5 antibodies.
GlyR: Glycine receptors (GlyRs) are ligand-gated ion channels that enable fast synaptic neurotransmission in the spinal cord and brainstem. Defects in the GlyR life cycle, such as increased degradation, are associated with various neurological diseases [59]. The development of anti-GlyR antibodies is proposed to lead to enhanced receptor internalization and degradation and is associated with the autoantibody mediated form of stiff-person syndrome spectrum disorder (SPSD) [16,17,60]. SPSD is characterized by combinations of stiffness, rigidity, and painful muscle spasms. GlyR antibodies are also associated with spinal and brainstem disorders, and many patients have progressive encephalomyelitis with rigidity and myoclonus (PERM) [17]. About 53% of patients with GlyR antibodies also have coexisting GAD65 antibodies. Screening for GlyR antibodies can be done using CBA on transfected Human Embryonic Kidney (HEK) 293 cells [16].
MOG: Antibodies directed against Myelin Oligodendrocyte Glycoprotein (MOG) have been associated with optic neuritis (ON), myelitis and brainstem encephalitis, as well as with Acute Disseminated Encephalo Myelitis (ADEM)-like presentations [61]. Recent studies using new-generation CBA have demonstrated this association however MOG antibodies were previously thought to be involved in Multiple Sclerosis (MS). While MS and MOG encephalomyelitis have some clinical and radiological overlap, MOG-IgG-associated encephalomyelitis is its own diseases entity due to the potentially pathogenic impact of MOG-IgG, discrete histopathological features, and differences in clinical and paraclinical presentation, and treatment response/prognosis [61]. The diagnosis of MOG-associated disease (MOGAD) is proposed based on the following criteria: monophasic or relapsing acute ON, myelitis, brainstem encephalitis, or encephalitis, or any combination of these syndromes, MRI or electrophysiological (visual evoked potentials in patients with isolated ON) findings compatible with CNS demyelination, seropositivity for MOG- IgG as detected by means of a CBA employing full-length human MOG as target antigen [61]. International comparative studies of different MOG-IgG1 assays have shown that live cell–based assays yield the highest specificity for MOGAD and serum is the recommended specimen of choice. CBA must employ full-length human MOG as target antigen, and the use of Fc-specific (or IgG1- specific) secondary antibodies is highly recommended to avoid cross-reactivity with (specifically or non-specifically co-binding) IgM and IgA antibodies [61].
Ganglionic Acetylcholine Receptor: Autoantibodies to the nicotinic ganglionic acetylcholine receptor (α3-AChR) cause subacute or insidious dysautonomia [19]. The detection of antibodies to α3- AChR aids the diagnosis of neurological autoimmunity and cancer and provides information about disease severity. High levels of anti- AChR antibodies are associated with profound pandysautonomia while low levels are consistent with limited dysautonomia [19]. In addition to being a useful serologic marker for establishing a diagnosis of autoimmune autonomic neuropathy, a study has shown that that α3-AChR antibodies may be effectors of autonomic dysfunction in patients with idiopathic or paraneoplastic autonomic neuropathy [62].α3-AChR antibodies had been first detected using a Radio Immuno Precipitation Assay (RIPA) with quantification by means of labeling with 125I-α-bungarotoxin. ELISA assays have since been developed with comparable sensitivity and specificity to the RIPA.
Intracellular Antigenic Targets: Biomarkers in Paraneoplastic and Non-paraneoplastic Syndromes
Paraneoplastic syndromes are autoimmune disorders associated with cancer not related to metastases or side effects of therapy. Onconeural antibodies help aid in the diagnosis of paraneoplastic syndromes, as they are typically associated with specific tumor types and can help inform malignancy screening. It is important to test broadly for antibodies because patients with paraneoplastic disorders could exhibit multiple antibodies. Cancer treatment potentially with adjuvant immunotherapy is important in the treatment of Paraneoplastic Neurological Disease (PND). Often autoantibodies identified in PND, the location of the antigens is intracellular, either predominantly cytoplasmic, or nuclear [63]. We will discuss intracellular antigenic targets involved in several neurologic syndromes, and highlight diagnostic strategies for the autoantibodies involved.
Neurochondrin: Neurochondrin is a 75-kDa protein specifically expressed in neurons. Neurochondrin, has been shown to regulate neural outgrowth, synaptic plasticity and dendritic morphogenesis [64-66]. Neurochondrin is highly expressed in cerebellar Purkinje cells, brainstem, and lateral parts of the central amygdala nuclei, hippocampal pyramidal cells, and autonomic and peripheral nervous systems. Neurochondrin has been identified as an intracellular neuronal antigen targeted by specific autoantibodies in autoimmune cerebellar degeneration [66]. Patients with cerebellar degeneration who did not respond to antibody-depleting treatments did improve with long-term immunosuppressive T-cell directed treatments while antineurochondrin titers in CSF and serum stayed unchanged [66]. This suggests that T cells, rather than B cell- derived antibodies, are likely to contribute to disease pathogenesis. In another study, neurochondrin autoimmunity was associated with non-paraneoplastic rapidly progressive rhombencephalitis with poor neurologic outcomes [67]. However paraneoplastic cases may still occur and a uterine carcinoma has been identified in one patient [67].
Homer-3: Homer-3 is a constitutively expressed member of the Homer family of postsynaptic density (PSD) scaffolding proteins. It is expressed in high levels in PCs and enriched in the dendritic spines. It is also found in the somata and PC axons [68]. Homer-3 autoimmunity is associated with a rare cerebellar ataxia. When tested by immunohistochemistry (IHC) using cerebellum tissue sections, Homer-3 antibodies given rise to a unique staining pattern of Purkinje cell (PC) somata and dendrites resembling a Gorgon head and are therefore often referred to as ‘Medusa head’ antibodies [30]. Anti-Homer-3 autoantibodies can be detected using IIF on unfixed or formalin-fixed frozen sections of mouse, rat or primate cerebellum tissue. Several antigen-specific assays are available for the detection of Homer-3 including, a CBA using Homer-3 expressing HEK293 and immunoblots. Homer-3 autoimmunity has also been found to be related to lung cancer in one patient, but only 2 cases have been published so far [30].
ITPR1: Inositol 1,4,5 triphosphate (ITPR1) is thought to be primarily an intracellular antigen located in membranes encompassing the endoplasmatic and, in muscle cells, sarcoplasmatic reticulum. ITPR1 is a ligand-gated calcium channel that modulates intracellular calcium signaling following stimulation by inositol 1,4,5-trisphosphate. ITPR1 autoimmunity has been associated with liver cancer, lung cancer, melanoma, and lymphoma tissue by IHC as well as in a number of tumor cell lines, suggesting a paraneoplastic etiology [69]. In one study, ITPR1 was found to be the target antigen of serum reactivity to Purkinje cells in patients with autoimmune cerebellar ataxia [21]. Identification of ITPR1 as the target antigen was first done by IFA and dot blot. As further confirmation, the PC antibody-positive sera and controls were then analyzed by an RC-IFA using HEK293 expressing murine ITPR1 and mock- transfected HEK293 [21]. Specific assays are needed to differentiate anti-ITPR1 from other PC autoantibodies in general due to similarities in tissue staining. The pathogenic nature of anti-ITPR1 is unknown. However, there is some indirect evidence for a potential pathogenic role [21]. Anti- ITPR1 antibodies can be detected by indirect immunofluorescence test (IIFT) using snap-frozen cerebellum sections. Antibodies will bind to the entire dendritic tree in the cerebellar molecular layer including the dendritic spines to the PC somata in the cerebellar PC layer, to the PC axons in the granular layer and the white matter and to the axonal terminals in the deep cerebellar nuclei [70]. Like Homer-3, anti-ITPR1 antibodies are considered to be ‘medusa head antibodies’ [30]. Antigen specific assays for anti-ITPR1 have also been developed including a CBA and a dot-blot assay.
NIF: Neuronal intermediate filaments (NIF) are cytoskeletal structural proteins (10 nm diameter) required for axon radial growth. They are heteropolymers consisting of 4 types of subunits defined by their molecular weight: neurofilament light chain (NfL, 68 kDA), neurofilament medium chain (NfM, 150 kDa), neurofilament heavy chain (NfH, 190-210 kDA), and either α-internexin in the central or peripherin in the peripheral nervous system. In the event of axonal damage NIF are released, into the CSF and subsequently into the blood. Neuronal intermediate filament (NIF) antibodies have been reported among patients with various diseases and healthy controls. Anti-NfM autoantibodies have also been found in patients with anti-NMDAR encephalitis, indicating underlying neuronal damage in these patients [71]. Antibodies against NIF are tested for by either a single assay type such as Western blot or ELISA, or by screening of neural antibodies by IFA with confirmation of NIF specificity by CBA [24]. The optimal diagnostic specificity is achieved by screening with tissue IFA and subsequent molecular confirmation by CBAs using a NIF-IgG profile that includes NfL-IgG [24]. Currently, the evaluation of CSF in addition to serum appears to improve testing sensitivity.24 Patients exhibiting NfL-IgG have CNS paraneoplastic autoimmunity. In one study, cancers were detected in 77% of patients and were most commonly of neuroendocrine lineage (49%) [24].
GFAP: Glial Fibrillary Acidic Protein (GFAP) is an intermediate filament protein expressed in the CNS by astrocytes. GFAP acts as an intracellular structural component of the astrocytic cytoskeleton. IgG autoantibodies against GFAP was first found in the CSF and serum of 103 patients undergoing testing for potential autoimmune neurologic disorders [72]. Autoantibodies to GFAP were found to be associated with a disabling and relapsing corticosteroid- responsive meningoencephalitis, with or without myelitis. Serological identification of GFAP autoantibodies is used for the detection of relapsing autoimmune meningoencephalomyelitis which is immunotherapy responsive and allows for a differential diagnosis [25,72]. The detection GFAP-IgG is completed by tissue-based indirect tissue IFA and confirmation by a CBA using a GFAP-transfected cell line. Patients with autoimmune GFAP meningoencephalomyelitis have overlapping autoimmune disorders and autoantibodies. Autoantibodies that occur along with anti-GFAP include GAD65, thyroperoxidase-specific IgG, P/Q-type voltage-gated calcium channel antibody, NMDAR– specific IgG, and antinuclear antibody [72]. A novel case of NIF IgG positivity in a patient with GFAP astrocyopathy has also been reported [73]. One-third of anti-GFAP cases are paraneoplastic, with ovarian teratoma being the most common malignancy [72]. Ovarian teratoma is best predicted when both NMDA-R-IgG and aquaporin-4-IgG coexist (71%) [25].
ARHGAP26: Antibodies to Rho GTPase activating protein 26 (ARHGAP26) bind specifically to the inner membrane and cytoplasm of Purkinje cell somata, dendrites and axons. Anti- ARHGAP26 antibodies belong mainly to the IgG1 subclass, and are produced intrathecally [29]. ARHGAP26 is also known as GTPase Regulator Associated with Focal Adhesion Kinase (GRAF).28 Antibodies to ARHGAP26 are found in the serum and CSF in patients with subacute autoimmune cerebellar ataxia [28,29]. Ovarian cancer has been found in one anti-ARHGAP26 positive patient with cerebellar ataxia suggesting a possible paraneoplastic etiology in some cases [28]. To detect antibodies directed against ARHGAP26, immunohistochemistry is performed on cryosections of adult mouse, monkey and rat tissue sections to identify reactivity of CSF and serum to structures in the molecular layer (ML), the Purkinje cell layer (PCL) and the white matter (WM) of the cerebellum. Specific binding to PC can be confirmed by double labeling of PCs with the patient antibody and an anti-calbindin antibody [29].
KLHL11: Antibodies to kelch-like protein 11 (KLHL11) were first identified in a 37-year-old man with a history of seminoma presented with vertigo, ataxia, and diplopia [74]. KLHL11 autoantibodies have been found to be a biomarker of testicular germ-cell tumor and PND, often refractory to treatment. The common presenting feature of KLHL11 encephalitis is a rhombencephalitis phenotype with ataxia, diplopia, dysarthria, vertigo, hearing loss, and tinnitus [23]. An immunopathologic association is supported by the strong oncologic connection, KLHL11-specific T-cell response, human leukocyte antigen associations, and brain histopathology. The detection of KLHL11-specific IgG is a useful tool for the accurate identification of this disorder. Brain MRIs of patients with KLHL11 encephalitis are often abnormal (76%) with T2/FLAIR hyperintensities found commonly in the temporal lobes, brainstem, cerebellum, and diencephalon with or without gadolinium enhancement [23]. Often atrophy, involving the cerebellum, medial temporal lobes, or globally, can be found chronically as well as hypertrophic olivary degeneration. Autoantibodies to KLHL11 can be detected by CBA screening with subsequent tissue-based IFA confirmation. In one study, all patients with KLHL11 encephalitis yielded a positive and highly stereotyped Sparkles tissue-based IFA pattern [23].
DNER: Anti-Tr antibodies has been identified in patients with subacute and severe cerebellar ataxia and 89% of such patients are also exclusively found to be diagnosed with Hodgkin’s Lymphoma (HL) [31]. The target antigen of anti-Tr antibodies is the Delta/ Notch-like epidermal growth factor-related receptor (DNER). While the main epitopes of anti-Tr have been mapped to the extracellular region of DNER, DNER has also been detected intracellularly in the sorting endosomal compartment of dendrites and cell bodies in various types of CNS neurons [30]. Anti-Tr autoantibodies are characterized based on a well-defined immunostaining pattern of punctate immunoreactivity in both the dendritic tree and soma of Purkinje cells but not in their axons [75]. Anti-Tr is commonly associated with HL and is very frequently associated with cancer. A standardized recombinant cell-based indirect immunofluorescence assay (RC-IFA) has been developed for the detection of antibodies against DNER [76]. Using well-defined anti-Tr–positive sera and controls, this new DNER assay was found to have a high sensitivity (100%) and specificity (100%) and perform better than the classic IFA using dried mammalian cerebellar cryosections commonly used in laboratories which has a sensitivity of (89.5%) and specificity of (100%) [77].
The importance of early detection of neurological autoantibody biomarkers allows for the recognition of potentially immunotherapy-responsive neurological syndromes and possible detection of otherwise clinically occult tumors at an early and highly treatable stage. While the identification of new disease- specific autoantibodies has facilitated diagnosis, there remain large gaps in our understanding of the underlying immune responses and pathophysiology. Further identification of biomarkers that facilitate our understanding of the underlying immune mechanisms and that are correlated to treatment response would be highly valuable towards predicting the progression of autoimmune neurologic diseases and allow for disease monitoring. Understanding the immune profile and inflammatory signature may help inform future therapeutic trials by identifying the best immunotherapy based on targeted mechanisms of action. However, as a single biomarker rarely is the determinant of any disease, early consideration during the development and validation phase are required to provide reliable analysis. Additionally, the identification of specific immune profiles related to disorders resulting from environmental exposures may provide an opportunity to identify variations in immune biomarkers over time. Currently, the combination of clinical, radiographic, and presentations and laboratory neural autoantibody testing are fundamental in the diagnosis of autoimmune neurological disorders. The expanding field of autoantibody-mediated autoimmunity has allowed for the identification of several anti-neuronal and anti-glial autoantibodies which aid in the diagnosis of specific neurological syndromes. Antibody screening has evolved to be a vital tool in the diagnosis and management of these neurological syndromes and can be done using IFA on cell substrates and tissue mosaics. However, diagnostic testing must be interpreted in the context of the clinical presentation. The past decade has brought a great number of reports of neuronal autoantibodies in conjunction with clinical observations. There is now a need for additional research to understand general mechanisms of autoimmunity to address questions regarding immune checkpoint dysregulation, antibody pathogenicity, and target-specific immunotherapy [78].
The diagnosis and prognosis of neurological diseases can be facilitated by detection of neuronal autoantibodies. In addition to the identification of neuronal autoantibodies, clinical symptoms and radiographic findings must be considered to determining a final diagnosis. For the detection of neuronal autoantibodies to either synaptic/neuronal cell surface or intracellular antigens, commercial assays are available. IFA using recombinant cell or tissue substrates have been found to be a useful tool for antibody screening. Using an integrated approach to identify underlying mechanisms of autoimmunity will aid clinicians in the determination of optimal therapeutic strategy.
IH and IV are employees of EUROIMMUN US, a company that develops and manufactures assays for the detection of disease- associated antibodies.
[CrossRef] [Google Scholar] [PubMed] [Publisher].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
[CrossRef] [Google Scholar] [PubMed].
Citation: Venkataraman I, Heckler I, Piquet AL (2022) The Testing Landscape of Autoimmune Neurological Conditions: Newly Discovered Cell Surface and Intracellular Antigens. J Clin Chem Lab Med. 5:211.
Received: 07-Mar-2022, Manuscript No. JCCLM-22-16175; Editor assigned: 10-Mar-2022, Pre QC No. JCCLM-22-16175 (PQ); Reviewed: 24-Mar-2022, QC No. JCCLM-22-16175; Revised: 31-Mar-2022, Manuscript No. JCCLM-22-16175 (R); Published: 07-Apr-2022 , DOI: 10.35248/JCCLM.22.05.211
Copyright: © 2022 Venkataraman I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.