International Journal of Advancements in Technology
Open Access
ISSN: 0976-4860
ISSN: 0976-4860
Research Article - (2015) Volume 6, Issue 1
Glaze making in some developing countries has not been encouraging, therefore making the act of glazing going into extinct. This setback is due to unavailability of required fluxes for formulating a workable low temperature transparent glaze. Meanwhile glass of different properties like Grade B borosilicate and Grade A borosilicate glasses which contain the required silica/flux ratio for formulating a low temperature transparent glazes are found to be abundantly available as waste, which are discarded and therefore polluting the immediate environment. This paper therefore discuss the availability and utilization of this particular type of waste glasses for formulating transparent glazes by processing and sieving the waste glasses into its finest particle called cullet. This processed cullet was used as a source of flux/silica with kaolin supplying the alumina for stabilizing the glaze. Cullet was composed in several proportions with kaolin in ratio 1-10 and vice versa. The composed glazes were fired at different temperature and atmosphere to detect the best condition at which cullet can be utilized as a source of silica/flux in formulating a transparent glaze.
<Keywords: Cullet; Glaze; Biaxial blend
In making ceramics, silica is found to be very important both at biscuit and gloss temperature since it forms the principal material needed for sintering or conversion to glassy state. During glass production, the principal raw material used is silica which comes in several compounds of silicates, melted and formed to shape at high temperature, with addition of several compounds called fluxes a catalyst that accelerates the melting temperature of the silica at fairly lower temperature. Youssef, Abadir and Shater note that manufacturing process of ceramics comprises excavation of raw materials which is mixed together in different ways to form bodies and glazes. It is the interaction between the bodies and glazes after firing that brings about its attractiveness and properties which make it useful to man in every ramifications of life [1]. Similarly such raw materials are excavated and used for glass processing and production. The waste from glass formation processes and used glasses and cullets are recycled for further production in to pulverized fine grains.
Cullet is a name given to waste broken glass which contains the major materials required for glaze preparation. Glaze is described according to Carty as a special sort of glass differing from windowglass and glass ware in its lower thermal expansion and higher alumina content, which increase its viscosity and help it to adhere to the clay body [2]. Most Cullet consists mainly of silicon, sodium, and calcium oxides (referred to as soda-lime-silica glass) with other minor components, such as aluminium and magnesium oxides just to improve its viscosity.
Flux is a term applied to those compounds that lower the melting point of a glaze; although many chemicals with a low melting point will also readily combine with silica to form a glassy crystal. Ryan describes flux as a material which lowers the fusion temperature of the mixture to which it is added [3]. But getting the required flux for making glaze could be difficult sometimes since available fluxes for producing leadless low temperature glazes are imported at colossal rate and thorny to get for contemporary studio potters and ceramic students. Meanwhile all the essential fluxes needed in making glaze are also used for making glasses which have turn to waste materials in the environment.
The potential of replacing natural fluxes with cullet has been reported in Youssef and Tarvornpanich [4,5]. This underscore the importance of recycling cullet which will not only turn waste to wealth but will also reduce the stress and cost of seeking fluxes for ceramic glazes. Glass is found to be in different types and properties and getting their cullet for exploration will not be too difficult since cullet is more of a nuisance to the environment. For the purpose of this research, exploring the composition and effect of cullet of different properties will be the major means of flux derivation. In this direction, the use of cullet for total or partial replacement of fluxes in ceramics glazes will be a very promising initiative as it will strongly contribute to sustainable development of ceramics industries in Nigeria, enhance the use of and also sustain the environmental benefits of the society.
Overview of ceramic glazes
In the last four decades, intense research work has gone into ceramic glazes development aimed at a better knowledge and understanding of glazes materials and their properties, therefore widening the art of glaze usage and application. Fournier define glaze as a sort of glass differing from window-glass and glass ware in its lower thermal expansion and higher alumina content, which increases its viscosity and help it to adhere to the body [6]. Irabor also describes glaze as a mixture of complex silicates and borates which is applied on ceramic products before heat treatment to produce the glossy-smooth or matty finish as desired [7]. This therefore means that without silica, there will be no glass or glaze. Cardew states that the most important glass formers are silicon (valency 4) boron (valency 3) and phosphorus (valency 5) [8]. He further explains that the structure of pure silica glass is thought to be a random network of silicon and oxygen continued indefinitely in three dimensions without any regular repetition. These solid bonds are very strong and certainly require a very high temperature to loosen it bonds and make it melt. According to Nelson silica commonly called Flint is the essential glaze ingredient, it is also known as quartz in its pure crystalline state [9].
In theory of glass structure silica is regarded as the network former while the fluxing oxides (elements of lower valencies) as network modifier because they break the silicon-oxygen bond and therefore lowering the melting temperature. Generally glass makers like their melt to be fairly fluid, so it can be manipulated and they therefore use only a small quantity of alumina in the batch but ceramist need a viscous melt to prevent the glaze from trickling off the surface of their pots and they consequently use much more of it.
Borosilicate glass
Borosilicate glass is a type of glass with the main glass-forming constituents’ silica and boron oxide. Borosilicate glasses are known for having very low coefficients of thermal expansion (~5 × 10−6/°C at 20°C), making them resistant to thermal shock, more so than any other common glass.
Borosilicate glass is made mainly of silica (70-80%) and boric oxide (7-13%) with smaller amounts of the alkalis (sodium and potassium oxides) and aluminium oxide. This type of glass has relatively low alkali content and consequently has good chemical durability and thermal shock resistance (it doesn’t break when changing temperature quickly). As a result it is widely used in the chemical industry, for laboratory apparatus, for ampoules and other pharmaceutical containers.
In addition to the quartz, sodium carbonate, and calcium carbonate traditionally used in glassmaking, boron is used in the manufacture of borosilicate glass so as to affect it properties for a preferred use. Boron is a very strong flux in low temperature ceramic glazes and performs the same function in glass production.
Typically, the resulting glass composition is about
70% silica,
10% boron oxide,
8% sodium oxide,
8% potassium oxide, and
1% calcium oxide (lime).
Though somewhat more difficult to make than traditional glass (Corning conducted a major revamp of their operations to make it), it is economical to produce; its superior durability, chemical and heat resistance finds excellent use in chemical laboratory equipment, flasks, beakers and oven proof cookware, lighting and in certain cases, windows.
In order to avoid mixture of other impurities with the cullet, care was taken in processing the injection vial to cullet; this was done with thorough washing of the glass so as to remove every dirt which might serve as impurities in the cullet. The cullet was dried and pulverized ground and ball milled for several hours to make it finer and ready for sieving.
Making of test tiles
Small test tiles were made for the purpose of testing the glaze behaviours on the surface of the tiles. The green ware (Test Tiles) was allowed to dry completely before it was placed in the kiln. The spy hole was left opened for an hour so as to allow the moisture to escape.
Glaze composition in biaxial blend
In order to formulate suitable glaze from glass, having the idea that glass already contain the required silica and flux needed in formulating a glaze, biaxial blend of glaze composition was adopted to determine the best ratio at which cullet will combine with other materials to form a glaze.
The following factors were strictly adhered to during composition
1) Cullet was accurately measured using the three beam balance.
2) Cullet was mixed with the combining materials and sired very well with water been added for thorough mixing.
3) Every composed blend was labelled so as to avoid mix up of any kind.
4) Every produced test tiles was also labelled for proper accuracy after firin.
Temperature: 1100°C, 1200°C: Measuring instrument: Thermocouple
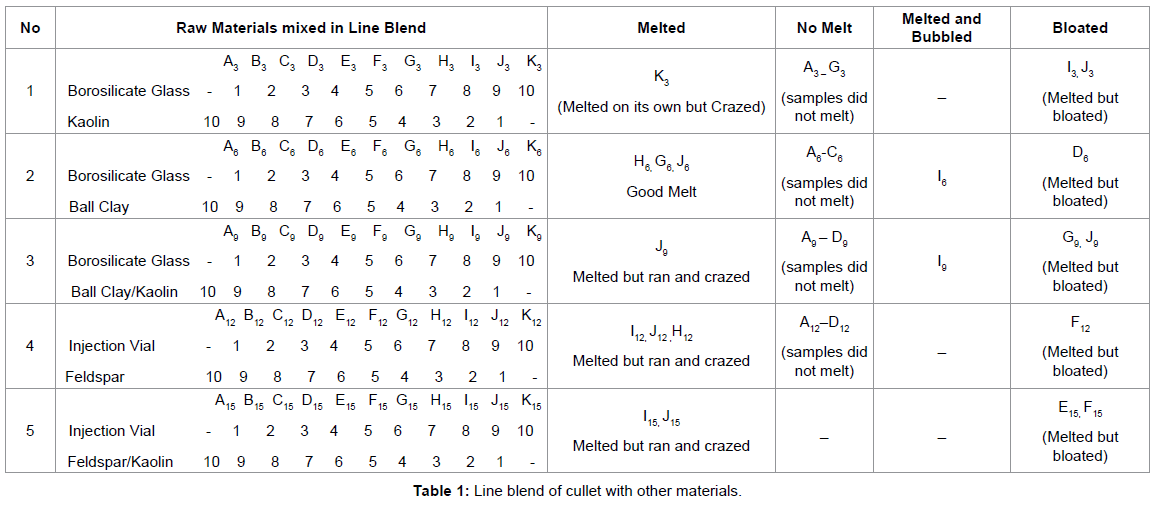
Table 1 Line blend of Cullet with other materials.
Cullet fired under different temperature and atmosphere
• First firing at 1100°C was under reduction atmosphere
• Second Firing at 1200°C was also under reduction atmosphere
• Third firing with substitution of Bari kin Ladi Kaolin with Auchi Kaolin and mixture of cullet with other oxides was done in reduction atmosphere
• Fourth Firing (recomposing some previous result and fire under oxidation and reduction atmosphere)
Observations after firing
It was observed that borosilicate glass formed a glassy transparent glaze with kaolin in ratio 10% of kaolin and 90% of borosilicate glass. It was observed that most of the composition made with other oxides melted but blistered and bloated. Feldspar kaolin and borosilicate glass gave a good result but crazed and ran. That rapid reduction firing under a gas kiln produces a bloating and bubbling effect with cullet glazes. From the listed observations, it was clearly seen that kaolin gave the best result with borosilicate glass at fairly low temperature.
The research has proved that desirable glazes can be made from cullet without addition of any other fluxes. The study has also proved that non-crazing and non-crawling or running glazes can be made from cullet at low temperature. It was also pragmatic from the research that borosilicate glass produced essential result when used as a source of silica/flux as compared with the common researches which have centered on the use of soda-lime silica glasses in forming ceramic glazes. The research furthermore showed the expediency of cullet in forming ceramic glazes with its viability as a replacement for frit in existing ceramic glaze batches, which will also reduce the melting temperature of the batch.