Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Review Article - (2020)Volume 9, Issue 2
There may be a bidirectional relationship between sleep and pain in patients with chronic pain. Actigraphy is increasingly being used as a non-invasive and objective method to assess sleep in chronic pain patients. This systematic review aimed to evaluate the utility of actigraphy in chronic pain patients. Additionally, meta-analyses were conducted to compare sleep parameters measured by actigraphy with those measured by sleep diary and polysomnography. Medline (1946-2019), Medline In-Process (May 2019), Embase (1947-2019), Cochrane Central Register of Controlled Trials (1991-2019), Cochrane Database of Systematic Reviews (2005-2019), and PubMed-NOTMedline (1946-2019) were searched for studies using actigraphy to measure sleep in chronic pain patients. Using the random effects model, meta-analyses were conducted to examine the concordance of actigraphy versus sleep diary and actigraphy versus polysomnography for commonly measured sleep parameters. Thirty-four studies with 3,590 patients were included. As an adjunct to sleep diary, actigraphy detected improvements in various sleep parameters after interventions in 10 studies and provided a useful objective sleep metric when comparing pain patients with healthy subjects in four studies; however, diary measurements were more “sensitive”. Comparing sleep diary versus actigraphy, sleep onset latency was significantly lower with actigraphy (mean difference of 22.7 minutes lower; 95% confidence interval: 13.2 to 32.2 minutes lower; p<0.01). No sleep parameters were significantly different between polysomnography and actigraphy; however, the confidence intervals were large. Actigraphy is an objective assessment tool that is being increasingly utilized to measure sleep in chronic pain patients. Based on studies that have measured sleep with both sleep diary and actigraphy, there are intrinsic differences between the two assessment methods as actigraphy lacks the cognitive component of subjective measures. Even though no differences in sleep parameters were detected between actigraphy and polysomnography, it cannot be established that the two are equivalent objective measures because of the limited number of studies and large variability.
Actigraphy; Sleep; Chronic pain
PSG: Polysomnography; TST: Total Sleep Time; SE: Sleep Efficiency; SOL: Sleep Onset Latency; WASO: Wake after Sleep Onset; RCT: Randomized Controlled Trial; CBT: Cognitive Behavioral Therapy
It is estimated that up to 50-80% of chronic pain patients report sleep disturbances [1-3]. The relationship between pain and poor sleep is not fully elucidated and likely bidirectional [4]. Limited research suggests that sleep has a greater impact on pain than pain on sleep [2,5]. It is important to assess sleep problems in chronic pain patients to treat their sleep and pain. The gold standard for sleep assessment is polysomnography (PSG); however, PSG is expensive and requires many physiological monitors [6]. A sleep diary is another tool that can measure sleep for longer periods, but they are subjective and can be cumbersome to complete [7].
Actigraphy is a non-invasive and objective method to assess sleep. It uses a small actigraph monitor, usually a wristwatch-like device that contains an accelerometer to measure motor activity over predefined periods (epochs) [8]. The activity in each epoch is then analyzed by computer software and defined as either “sleep” or “wake” [8]. Compared to PSG, actigraphy presents several advantages: 1) it allows for continuous monitoring from days to weeks; 2) it allows for monitoring in the patient’s normal sleep environment; 3) it is less invasive (i.e. patient only has to wear watch versus being attached to several monitors) ; and 4) it is of lower cost [8]. The Clinical Practice Guideline on Actigraphy developed by the American Academy of Sleep Medicine suggests using actigraphy as an adjunct for assessing sleep in insomnia and circadian rhythm sleep-wake disorder [9].
Sleep parameters that are commonly assessed by actigraphy, sleep diary, and PSG include total sleep time (TST; total duration of sleep during the major sleep period), sleep efficiency (SE; proportion of time the patient is asleep during the total time in bed), sleep onset latency (SOL; duration between getting in bed and falling asleep), and wake after sleep onset (WASO; duration of time awake after initial sleep onset and before getting out of bed) [10]. Although correlated, discrepancies exist between the different assessment methods [7]. In particular, these discrepancies have been demonstrated to be larger in certain patient populations, such as patients with insomnia and chronic conditions [11,12].
Actigraphy is a potentially promising tool to diagnose and treat sleep disorders in chronic pain patient populations; however, the use of actigraphy in this population has not been systemically examined. Additionally, the discrepancy between actigraphy versus sleep diary or PSG has not previously been evaluated. The primary objective of this systematic review is to qualitatively assess the utility of actigraphy in the following contexts: 1) evaluating sleep after an intervention, 2) investigating the relationship between sleep and pain, 3) assessing the relationship between sleep and physical activity, and 4) comparing sleep in pain patients with healthy subjects. The secondary objective is to conduct a meta-analysis to determine the discrepancy between actigraphy versus sleep diary and actigraphy versus PSG in chronic pain patients.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: 1) the participants were chronic pain patients (experiencing pain for ≥ 3 months) [13]; 2) actigraphy was used to measure sleep parameters; 3) the sleep measurement period was ≥ 5 days; 4) the participants were adults (>18 years old); 5) the studies included ≥ 15 participants (to exclude case studies and very small studies); and 6) the study was published in English. As this review is focused on the general chronic pain experience, the following types of pain conditions were excluded: 1) cancer; 2) spinal cord injuries; 3) traumatic brain injuries; 4) palliative conditions; 5) dysmenorrhea; 6) irritable bowel syndrome; 7) episodic headache, and 8) failed back surgery syndrome.
Search strategy
A literature search was designed by an information specialist (ME) and conducted on May 22, 2019 of Medline (1946-2019), Medline In-Process (May 2019), Embase (1947-2019), Cochrane Central Register of Controlled Trials (1991-2019), Cochrane Database of Systematic Reviews (2005-2019), and PubMed-NOTMedline (1946-2019). Search terms included “ chronic pain ” , “sleep”, and “actigraphy”. The complete strategy is presented in Supplemental Material 1. The citations of included studies and other review articles were also searched for additional studies.
Study selection
Titles and abstracts were independently screened by two authors (DA, JS). Following the selection of abstracts, the full texts of articles identified for possible inclusion were obtained and assessed for inclusion independently by two authors (DA, JS). Disagreements were resolved by consensus or by consulting a third author (FC).
Data extraction
Study characteristics were extracted independently by two authors (DA, JS) using a standard data collection form. The following information was extracted from each study: study characteristics, study findings, patient population, actigraphic information (device model, assessment period, device placement location, epoch, scoring, and measured sleep parameters), other measures of sleep (i.e. diary or PSG), and specific sleep data values used for meta-analysis.
Meta-analysis
Meta-analyses were performed to examine the concordance of: 1) actigraphy versus sleep diary and, 2) actigraphy versus PSG for TST, SE, SOL, and WASO by comparing the mean differences. The sleep parameter values were extracted from studies where more than one mode of sleep measurement was used (i.e., actigraphy along with sleep diary and/or PSG) and the parameters were reported. In interventional studies, only baseline data values were used. In studies with multiple groups, chronic pain patients were pooled together. All analyses were performed with Review Manager 5.3 software using the random effects model [14]. For quality assessment, observational studies were assessed using the Newcastle-Ottawa Scale (which was adapted for cross-sectional studies) [15,16] and randomized controlled trials (RCTs) were assessed using the Cochrane Risk of Bias Tool [17].
Search results
The search yielded 2,060 results, with 1,252 studies remaining after duplicates were removed. Screening of titles and abstracts provided 77 studies for full text review. After full text review, 34 studies with 3,590 patients were included for analysis (Figure 1) [18-51].
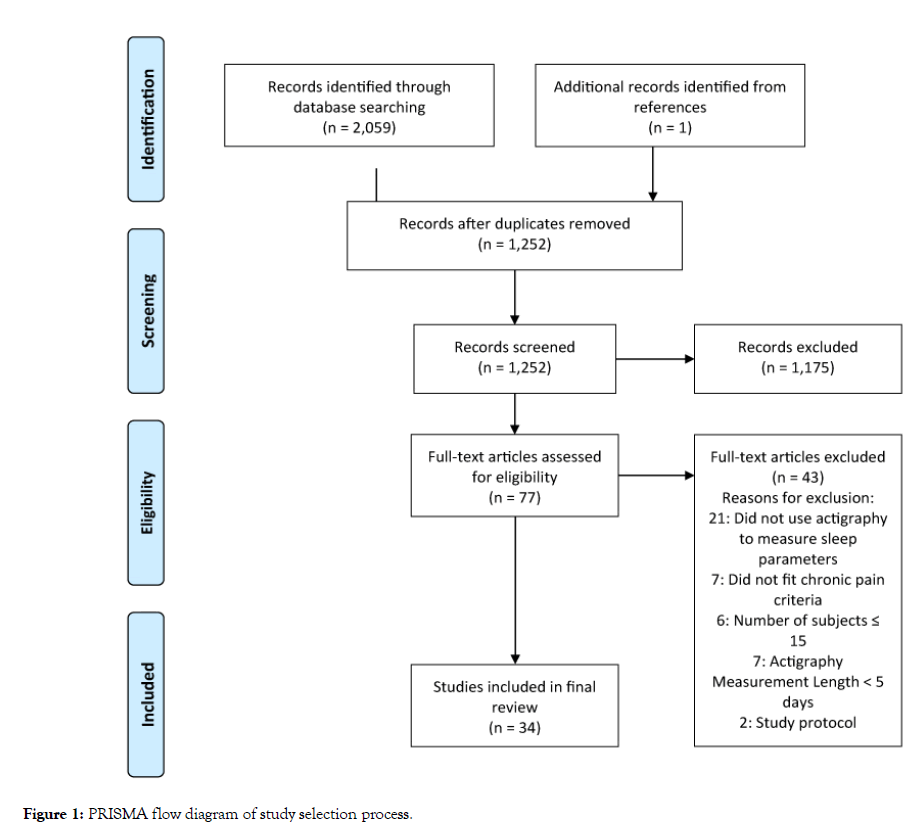
Figure 1: PRISMA flow diagram of study selection process.
Study characteristics
Twenty-five studies were observational studies, and nine studies were RCTs. Study locations included the United States (20), United Kingdom (5), Ireland (2), Spain (2), Norway (1), Australia (1), and Brazil (2). Ten studies were on fibromyalgia, eight on arthritis, and five on chronic back pain, two on chronic migraine, and nine on chronic pain patients in general. The study characteristics and findings are presented in Table 1.
Table 1: Study characteristics.
| Author/Year | Pain + Comorbid Conditions | Study Type/Main Objective | Total n/Groups | Age/Sex (%F) | Comparators | Key Findings |
|---|---|---|---|---|---|---|
| Evaluating Sleep after an Intervention | ||||||
| Blake et al. [18] | CP | Cohort: effect of CBT on sleep | 46/CBT (24); Control (22) | 47/63 | NA | At baseline, PSQI scores correlated with fragmentation index and SE. After CBT, actigraphy detected a decrease in wake bouts; no change with PSQI scores. |
| Edinger et al. [19] | FM + Insomnia | RCT: evaluation of CBT-I | 38/CBT (15); SH (16); UC (7) | 49/96 | Diary | CBT resulted in decreased TWT, SOL, and increased SE with diary and decreased SOL with actigraphy compared to control intervention. CBT resulted in less TST and SOL variability. |
| Smith et al. [20] | OA + Insomnia | RCT: effect of CBT-I on sleep and pain | 100/CBT-I (50); Control (50) | 59/79 | Diary, PSG | Compared with control, CBT-I demonstrated greater reductions in WASO and lower pain as measured by diary, actigraphy, and PSG. |
| Smitherman et al. [21] | CM + Insomnia | RCT: efficacy of CBT-I | 31/CBT-I (16), control (15) | 31/90 | NA | CBT-I led to greater TST and SE and reduced PSQI scores at follow-up. |
| Tang et al. [22] | CP + Insomnia | RCT: utility of hybrid treatment that targets insomnia and pain | 20/Hybrid treatment (10), control (10) | 49/90 | Diary | CBT led to reductions in TIB, SOL, and WASO measured by diary and actigraphy. Diary found increases in TST and SE; actigraphy found a decrease in TST and no change in SE. |
| Vitiello et al. [23] | OA + Insomnia | RCT: effect of CBT-PI, CBT-P, and control interventions on sleep and pain outcomes | 367/CBP-PI (122), CBT-P (122), Control (123) | 73/78 | NA | CBT-PI and CBT-P improved actigraphic SE vs. control intervention. CBT-PI improved ISI scores vs. both CBT-P and control. |
| McCrae et al. [24] | FM + Insomnia | RCT: effect of CBT-I and CBT-P | 113/CBT-I (39), CBT-P (37), control (37) | 53/97 | Diary, PSG | Diary SOL, WASO, SE, and SQ improved after intervention. No changes in actigraphic or PSG sleep parameters. |
| Burgess et al. [25] | CBP | Cohort: feasibility and effect of morning bright light treatment on pain, sleep, mood, and circadian timing | 25/NA | 48/27 | NA | While the intervention improved pain and improved SQ, actigraphy did not show any changes in TST and SE. However, actigraphy was able to detect phase advances in circadian timing, which was associated with reductions in pain. |
| Castano et al. [26] | FM | Cohort prospective: effect of melatonin supplementation on sleep | 33/NA | >40/100 | NA | Melatonin supplementation improved SQ and also actigraphic findings; however, PSQI scores started to show improvement at lower melatonin doses compared to actigraphic findings. |
| Eadie et al. [27] | CBP | RCT: feasibility of RCT for assessing the effectiveness of physiotherapy interventions on sleep | 46/Walking program (16), Exercise class (15), Usual Physiotherapy (15) | 45/62 | Diary | PSQI and ISI detected improvement after physiotherapy interventions; study did not assess differences in sleep parameters due to non-compliance. Baseline differences existed between sleep parameters: TST was 30 min longer and SOL was 10.4 min shorter with actigraphy compared to PSD. |
| Gozani et al. [28] | CBP | Cohort: if transcutaneous electrical nerve stimulation improves back pain | 554/NA | 55/53 | NA | Actigraphy showed increased TST and decreased PLM index post-intervention in patients who had pain improvement. |
| Investigating the Relationship between Sleep and Pain | ||||||
| Frange et al. [29] | CP + Insomnia | Cross-sectional: relationship between CP and sleep in postmenopausal women | 52/control (n = 10), CP (n = 12), Insomnia (15), CP + Insomnia (15) | >50/100 | PSG | Increased TST predicted higher pain upon waking, and higher pain at bedtime predicted increased TST. CP patients with comorbid insomnia had more sleep episodes and longer SOL compared to healthy subjects. |
| Salwen et al. [30] | OA + Insomnia | RCT: impact of sleep on pain within a trial of CBT-I | 74/NA | 60/77 | Diary | Both diary and actigraphic TST at mid-treatment were higher for pain responders vs. non-responders. |
| Tang et al. [31] | CP + Insomnia | Cross-sectional: bidirectional relationship between sleep and pain | 119/NA | 46/74 | NA | Presleep pain predicted poorer subjective SE but not SQ and actigraphic SE. SQ and subjective SE were predictive of less next-day pain; however, actigraphic SE was predictive of greater next-day pain. |
| Anderson et al. [32] | FM | Cross-sectional: if sleep can predict pain | 74/NA | 53/95 | Diary | No relationship between TST, TWT, and pain in FM. Comparing diary and actigraphy measures, TST was similar whereas TWT was greater with diary measures. |
| Liszka-Hackzell et al. [33] | CBP | Cross-sectional: bidirectional relationship between sleep and pain | 18/NA | 52/44 | NA | No bidirectional relationship between pain and sleep. |
| O'Brien et al. [34] | CP | Cross-sectional: daily variations in sleep and pain | 22/NA | 44/100 | Diary | Bidirectional relationship between sleep and pain detected with diary; however, no relationship was detected with actigraphy. |
| Parmelee et al. [35] | OA | Cross-sectional: racial differences in sleep and pain | 224/African American (96); non-Hispanic white (128) | 65/77 | NA | Compared to non-Hispanic whites, African Americans had decreased SE, increased WASO and sleep fragmentation, and more awakenings. Sleep parameters not associated with pain the day before or after. |
| Whibley et al. [36] | OA | Cross-sectional: whether sleep impacts next-day pain and fatigue | 160/NA | 71/62 | NA | Next-day pain and fatigue associated with SQ but not actigraphic sleep parameters. |
| Assessing the Relationship between Sleep and Physical Activity | ||||||
| McKenna et al. [37] | RA | Cross-sectional: sleep and physical activity | 32/NA | >50/66 | NA | TST positively correlated with physical activity. |
| Tang et al. [38] | CP + Insomnia | Cross-sectional: effect of sleep on next-day physical activity | 119/NA | 46/74 | NA | Higher SQ predicted higher physical activity the next day. Subjective and objective SE did not predict physical activity. |
| Andrews et al. [39] | CP | Cross-sectional: effect of physical activity on sleep | 50/NA | 54/60 | NA | Greater fluctuations in daytime activity associated with reduced TST. Higher average daytime physical activity and higher no. of reported pain sites associated with increased average awake time. |
| Comparing Sleep in Pain Patients versus Healthy Subjects | ||||||
| Lunde et al. [40] | CP | Cross-sectional: sleep in CP patients vs. Healthy Subjects | 72/CP (43), Healthy Subjects (29) | 68/79 | Diary | Diary and actigraphy reported that CP patients had increased TIB vs. healthy subjects. Diary also reported that CP had increased SOL and WASO. |
| Homann et al. [41] | FM | Cross-sectional: relationship of leptin and acylated ghrelin with sleep and pain | 33/FM (17), Healthy Subjects (16) | 40/100 | NA | Compared to healthy subjects, FM had later onset of sleep, delayed waking, increased TIB, reduced SE, and increased WASO and nocturnal sleep episodes. |
| Segura-Jimenez et al. [42] | FM | Cross-sectional: agreement between diary and actigraphic measurement | 180/FM (127); Healthy subjects (53) | NS/100 | Diary | Compared to healthy subjects, FM patients had greater actigraphic WASO. With PSQI, FM showed worse measurements in all parameters except TIB. PSQI estimated lower TST and higher SOL than actigraphy in FM; no differences in healthy subjects. |
| Robertso et al. [43] | CBP | Cross-sectional: sleep and the relationship with opioid analgesia | 31/CBP (21), Healthy subjects (10) | 44/52 | PSG | CBP patients displayed worse SQ and greater actigraphic TIB, TST, and SOL vs. healthy subjects; however, PSG did not detect a difference. Opioid usage did not affect actigraphic sleep parameters. |
| Ong et al. [44] | CM | Cross-sectional: sleep and circadian phase | 40/CM (20), Healthy subjects (20) | 32/100 | NA | Delayed sleep timing associated with more frequent migraine days. No differences in actigraphic sleep parameters found between CM and healthy subjects. |
| Other Studies | ||||||
| Chan et al. [45] | FM + Insomnia | Cross-sectional: sleep discrepancy of diary and actigraphic measurements | 223/NA | 52/93 | Diary | Average sleep discrepancies across all sleep parameters was small with no consistent direction; however, sleep discrepancy for any single night was large. Taking opioids was associated with greater night-to-night variability in WASO and TST. Diary and actigraphy estimates were mildly correlated with PSG values. |
| Mundt et al. [46] | FM + Insomnia | RCT: baseline concordance of diaries, actigraphy, and PSG and the ability of each to detect changes following CBT-I intervention | 113/CBT-I (39), CBT-P (37), control (37) | 53/97 | Diary, PSG | At baseline, diary recorded lower TST and SE vs. objective measures. For WASO, actigraphy a lower estimate while PSG a higher estimate vs. diary. Actigraphy estimated higher SOL and lower WASO vs. PSG. After CBT-I, diary detected improvements in SOL, WASO, and SE; actigraphy detected improvements in WASO and SE; and PSG found no improvements. |
| Okifuji et al. [47] | FM | Cross-sectional/ concordance of diary and actigraphic measurement | 75/NA | 46/97 | Diary | In FM, average actigraphic TST was less than diary TST. |
| Campbell et al. [48] | OA + Insomnia | Cross-sectional: sleep and pain and their association with INS | 208/OA + Insomnia (118), OA (31), Insomnia (30), Healthy Subjects (29) | 60/72 | Diary, PSG | Knee OA + insomnia patients had reduced SE based on diary, actigraphy, and PSG measures. Based on actigraphy, OA + insomnia patients had reduced SE vs. insomnia patients. TST, SOL, and WASO were worse than good sleepers with diary; however, findings were inconsistent with actigraphy and PSG. TST measured with actigraphy and PSG was low and same amongst groups. Objective measures showed large discrepancies in SE, TST, and WASO with diary for knee OA group. |
| Curtis et al. [49] | CP + Insomnia | Cross-sectional: relationship between sleep and cognition in INS, with or without CP | 60/CP (33), Healthy Subjects (27) | 70/67 | Diary | No differences in diary or actigraphic sleep parameters was found between CP patients and healthy subjects. In CP patients, diary WASO was associated with worse cognitive performance whereas actigraphic WASO was associated with better cognitive performance. |
| Curtis et al. [50] | FM + Insomnia | Cross-sectional: whether opioid dose and age predict discrepancy of diary and actigraphic measurement | 199/NA | 52/93 | Diary | Higher opioid doses increased SOL and SE discrepancy, with age affecting the direction of the discrepancy. |
| Goodchild et al. [51] | RA/Sjogren's syndrome | Cross-sectional: daily variations in fatigue and the relationship with sleep | 39/RA (25), SS (14) | 61/100 | Diary | Evenings of worse discomfort were followed by poorer SQ and SE. |
Abbreviations: FM: Fibromyalgia; CP: Chronic Pain; CBP: Chronic Back Pain; OA: osteoarthritis; RA: Rheumatoid Arthritis; CM: Chronic Migraine; CBT: Cognitive Behavioral Therapy; CBT-I: Cognitive Behavioral Therapy for Insomnia; CBT-P: Cognitive Behavioral Therapy for Pain; CBT-PI: Combined Cognitive Behavioral Therapy for Pain and Insomnia; RCT: Randomized Controlled Trial; NA: Not Applicable; PSG: Polysomnography; TST: Total Sleep Time; TWT: Total Wake Time; PSQI: Pittsburgh Sleep Quality Index; ISI: Insomnia Severity Index; SE: Sleep Efficiency; SQ: Subjective Sleep Quality; WASO: Wake after Sleep Onset; SOL: Sleep Onset Latency; PSD: Pittsburgh Sleep Diary; PLM: Periodic Leg Movement; TIB: Time in Bed.
Actigraph settings are presented in Table 2. Quality assessment of studies is presented in Table 3. Apart from two studies with poor quality [27,33], the remaining studies ranged from fair to good quality.
Table 2: Actigraph settings.
| Author/Year | Actigraph Device | Assessment Period/Device Location/Epoch | Software/Scoring | Actigraphic Parameters |
|---|---|---|---|---|
| Blake Et al. [18] | Actiwatch AW7A | 7 days x 3/NS/30 s | Actiwatch software (Sleep V7.27 analysis software b)/NS | TST, SE, SOL, WASO, fragmentation index; mean nighttime activity, number of wake bouts |
| Edinger et al. [19] | Actiwatch (Mini-Mitter Co, Inc, Sun River, Oregon, United States) | 14 days x 3/non-dominant wrist/NS | Actiwatch Sleep Analysis software/Medium sensitivity | TST, SE, SOL, WASO, TWT |
| Smith et al. [20] | Actiwatch 2 (Mini Mitter Co., Inc.) | 14 days x 5/non-dominant wrist/NS | NS/NS | TST, SE, SOL, WASO |
| Smitherman et al. [21] | Actiwatch 2 (Philips Respironics, Murrysville, PA, USA) | 14 days x 3/non-dominant wrist/30 s | Actiware software/NS | TST, SE |
| Tang et al. [22] | Actigraphy (then supplied by Cambridge Neurotechnology, Ltd) | NS/NS/NS | Actiwatch Activity and Sleep Analysis (Cambridge Neurotechnology Ltd., Cambridge, United Kingdom) version 5.43/NS | TIB, TST, SE, SOL, WASO |
| Vitiello et al. [23] | Actiwatch-2 (Respironics, Inc., Bend, Oregon, United States) | 7 days x 3/NS/NS | NS/TIB interval determined by diary | TST, SE, TWT |
| McCrae et al. [24] | Actiwatch 2 (Phillips Respironics, Bend, Oregon, United States) | 14 days x 3/non-dominant wrist/30 s | Actiware Sleep Analysis Software v.5.3.2/high sensitivity; TIB interval established by diary | TST, SE, SOL, WASO |
| Burgess et al. [25] | Actiwatch Spectrum (Respironics, Bend, Oregon, United States) | 20 days/non-dominant wrist/30 s | Actiware 6.0.9 program (Respironics, Bend, Oregon, USA)/TIB interval guided by event markers, light data, diary, and activity levels | TST, SE, sleep onset time, sleep offset time |
| Castano et al. [26] | Actiwatch (Cambridge Neurotechnology Ltd., Cambridge, United Kingdom) | 110 days/non-dominant wrist/NS | Sleep analysis (Cambridge Neurotechnology Ltd.) software package/NS | TIB, TST, SE, SOL, wake bouts, total nocturnal activity, immobility |
| Eadie et al. [27] | Actiwatch A4 | 7 nights/non-dominant wrist/10 s | Actiwatch software (Sleep V7.27 analysis software)/medium sensitivity | TST, SE, SOL, TWT, number of awakenings |
| Gozani et al. [28] | TENS device (Quell, NeuroMetrix Inc., Waltham, Massachusetts, United States) contained 3-axis MEMS accelerometer | 14 days x 2/upper calf/60 s | NS/Epochs classified as sleep if user was recumbent and mean activity was above threshold. Nights with total time <4h and >10h excluded | TST, SE, SOL, WASO, PLM |
| Frange et al. [29] | Motionlogger Watch (Ambulatory Monitoring, Inc., United States) | 10 days/non-dominant wrist/60 s | ActionW 2.6 software (Ambulatory Monitoring Inc.)/Zero crossing mode | TIB, TST, SE, SOL, WASO, sleep episodes (defined as awakenings that lasted for at least 1 min) |
| Salwen et al. [30] | Actiwatch 2 (Mini Mitter Co., Inc.) | 14 days x 5/non-dominant wrist/NS | NS/NS | TST, SE, SOL, WASO |
| Tang et al. [31] | Actiwatch-Insomnia Model (has unique pressure sensor) | 7 days/non-dominant wrist/NS | Actiwatch Activity and Sleep Analysis (Cambridge Neurotechnology Ltd., Cambridge, United Kingdom) version 5.43/ TIB interval determined by event marker and pressure sensor | TST, SE, SOL, WASO |
| Anderson et al. [32] | Actiwatch 2 (Phillips Respironics, Bend, Oregon, United States) | 14 days/non-dominant wrist/30 s | Actiware Sleep Analysis Software v.5.3.2/High sensitivity; TIB interval established by diary | TST, TWT |
| Liszka-Hackzell et al. [33] | Actigraph AW- 64 (Minimitter Inc.) | 5 nights/non-dominant wrist/60 s | Actiwatch Sleep Analysis software/TIB interval was assumed to be from 2400h to 0600 h | Actual Sleep Percentage, SE, Wake Bouts, Number of Min Immobile, Number of Immobile Phases and Movement/Fragmentation Index |
| O'Brien et al. [34] | Actiwatch-score (MiniMitter/Respironics, Inc.) | 14 days/non-dominant wrist/30 s | Actiware 5 software program (MiniMitter/Respironics, Inc.)/High sensitivity; TIB interval determined by diary | TST, SE, SOL, WASO |
| Parmeleeet al. [35] | Actigraph GTX-3 W | 6 days/dominant wrist/10 s | Actilife software/Cole-Kripke algorithm; TIB interval derived from acclerometer data | TST, SE, WASO, number of awakenings, fragmentation index |
| Whibley et al. [36] | Actiwatch-Score (Philips Respironics, Mini Mitter, Murrysville, Pennsylvania, United States) | 5 days/non-dominant wrist/15 s | NS/TIB interval established by corroborating diary with actigraphy activity | TST, SE, SOL, WASO |
| McKenna et al. [37] | SenseWear Pro3 Armband (Bodymedia Inc., Pittsburgh, Pennsylvania, United States) | 7 days/arm/60 s | SenseWear Armband software/determines sleep/wake using variations in movement, changes in heat flux and skin temperature, and galvanic skin response | TST |
| Tang et al. [38] | Actiwatch-Insomnia Model (has unique pressure sensor) | 7 days/non-dominant wrist/30 s | Actiwatch Activity and Sleep Analysis (Cambridge Neurotechnology Ltd., Cambridge, United Kingdom) version 5.43/ TIB interval determined by event marker and pressure sensor | SE |
| Andrews et al. [39] | GT3X ActiGraph (ActiGraph, Pensacola, Florida, United States) | 5 days/NS/60 s | ActiLife software version 4.4.1/Sadeh algorithm; TIB interval determined by diary | TST, SE, number of awakenings, average wake time |
| Lunde et al. [40] | Actiwatch Plus (Cambridge Neurotechnology Ltd, Cambridgeshire, United Kingdom) | 14 days/non-dominant wrist/30 s | Actiwatch software (Actiwatch Sleep Analysis 2001 software, Version 1, 19; Cambridge Neurotechnology Ltd, Cambridgeshire, United Kingdom)/NS | TIB, TST, SE, SOL |
| Homann et al. [41] | Basic Motionlogger-L model (Ambulatory Monitoring Inc., Ardsley, New York, United States) | 8 days/non-dominant wrist/60 s | NS/Proportional integrating measure; TIB interval determined by diary | TIB, TST, SE, WASO, sleep onset time, sleep offset time, mean nocturnal activity, nocturnal wake episodes |
| Segura-Jimenez et al. [42] | SenseWear Pro3 Armband (BodyMedia Inc, Pittsburgh, Pennsylvania, United States) | 7 days/right upper arm/NS | SenseWear Professional software version 6.1/threshold of 95% “on-body” time was used to include an individual | TST, SOL, WASO (frequency, total duration and average duration), deep sleep and light sleep, sleep quality score (deep sleep/TST) |
| Robertson et al. [43] | Actiwatch-L (CamNTech Ltd., Cambridge, United Kingdom) | 14 days/NS/NS | Actiwatch Activity and Sleep Analysis’ software (CamNtech Motionware version 1.1.3; Cambridge Neurotechnology, Cambridge, United Kingdom)/TIB interval established by diary | TST, SE, SOL, WASO, fragmentation index, interdaily stability, intradaily variablility |
| Ong et al. [44] | Actiwatch Spectrum (Respironics, Bend, Oregon, United States) | 7 days/non-dominant wrist/60 s | Respironics Actiware ver- sion 6.0/medium sensitivity; TIB interval guided by event markers and diary | TST, SE, SOL, WASO, early morning awakening, sleep onset time, sleep midpoint time, sleep offset time |
| Chan et al. [45] | Actiwatch 2 (Phillips Respironics, Bend, Oregon, United States) | 14 days/non-dominant wrist/30 s | Actiware Sleep Analysis Software v.5.3.2; TIB interval established by diary corroborated with light data and activity level | TIB, TST, SE, SOL, WASO |
| Mundt [46] | Actiwatch 2 (Phillips Respironics, Bend, Oregon, United States) | 14 days x 3/non-dominant wrist/30 s | Actiware Sleep Analysis Software v.5.3.2/high sensitivity; TIB interval established by diary | TIB, TST, SE, SOL, WASO |
| Okifuji et al. [47] | Micro Mini Motionlogger Actigraph (Ambulatory Monitoring, Ardsley, New York, United States) | 7 days/non-dominant wrist/60 s | Action series (Ambulatory Monitoring Inc)/ Cole-Kripke algorithm; zero-crossing mode | TST, SE, SOL, WASO, mean sleep episode, activity during sleep |
| Campbell et al. [48] | Actigraph 2 (Philips HealtHSare/ Respironics) | 14 days/non-dominant wrist/60 s | NS/medium sensitivity | TST, SE, SOL, WASO |
| Curtis et al. [49] | Actiwatch-L (Mini Mitter Co., Inc.) | 14 days/non-dominant wrist/30 s | Actiware-Sleep v.3.3; TIB interval established by diary | TST, SE, SOL, WASO |
| Curtis et al. [50] | Actiwatch 2 (Phillips Respironics, Bend, Oregon, United States) | 14 days/non-dominant wrist/30 s | Actiware Sleep Analysis Software v.5.3.2; TIB interval established by diary, corroborated with light data and activity level | TIB, TST, SE, SOL, WASO, TWT |
| Goodchildet al. [51] | Actiwatch- Score (Cambridge Neurotechnology Ltd., Cambridge, United Kingdom) | 35 days/NS/30 s | Actiwatch Activity and Sleep Analysis v.5.32 software (Cambridge Neurotechnology Ltd., 2003)/NS | TST, SE, SOL, TWT |
Abbreviations: s: Second; m: Min; h: Hour; NS: Not Stated; TIB: Time in Bed; TST: Total Sleep Time; TWT: Total Wake Time; SE: Sleep Efficiency; SOL: Sleep Onset Latency; WASO: Wake after Sleep Onset.
Table 3: Quality Assessment of Studies.
| Cochrane Risk of Bias Tool for Randomized Controlled Trials | |||||||||
| Study | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other bias | Overall Risk of Bias | ||
|---|---|---|---|---|---|---|---|---|---|
| Random Sequence Generation | Allocation Concealment | Blinding of Participants & Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | ||||
| Eadie et al. [27] | Low | Low | Unclear | Low | High | Low | High | High | |
| Edinger et al. [19] | Unclear | Unclear | Low | Low | Unclear | Unclear | Low | Unclear | |
| McCrae et al. [24] | Low | Unclear | Unclear | Low | Low | Low | Low | Low | |
| Mundt et al. [46] | Low | Unclear | Unclear | Low | Low | Low | Low | Low | |
| Salwen et al. [30] | Low | Unclear | Low | Low | Low | Unclean | Low | Low | |
| Smith et al. [20] | Low | Unclear | Low | Low | Low | Unclean | Low | Low | |
| Smitherman et al. [21] | Low | Low | Low | Low | Low | Low | Unclear | Low | |
| Tang et al. [22] | Low | Low | Low | Low | Low | Unclean | Unclear | Low | |
| Vitiello et al. [23] | Low | Low | Low | Low | Low | Low | Low | Low | |
| Newcastle-Ottawa Scale for Cross-Sectional Studies | |||||||||
| Study | Selection | Comparability | Outcome | Total Score | |||||
| Representativeness of the sample | Sample size | Non-respondents | Ascertainment of Exposure | Assessment of outcome | Statistical test | ||||
| Anderson et al. [32] | * | - | - | ** | - | ** | * | 6 | |
| Andrews et al. [39] | * | - | * | ** | * | ** | * | 8 | |
| Campbell et al. [48] | * | - | - | ** | ** | ** | * | 8 | |
| Chan et al. [45] | * | - | - | ** | ** | * | 6 | ||
| Curtis et al. [49] | * | - | - | * | ** | ** | * | 7 | |
| Curtis et al. [50] | * | - | - | * | ** | ** | * | 7 | |
| Frange et al. [29] | - | - | - | ** | ** | ** | * | 7 | |
| Goodchild et al. [51] | - | - | - | ** | ** | ** | * | 7 | |
| Homann et al. [41] | * | - | - | ** | ** | ** | * | 8 | |
| Liszka-Hackzell et al. [33] | - | - | - | - | - | ** | - | 2 | |
| Lunde et al. [40] | * | - | - | ** | * | ** | * | 7 | |
| McKenna et al. [37] | * | - | - | ** | ** | ** | * | 8 | |
| O'Brien et al. [34] | * | - | - | * | ** | ** | * | 7 | |
| Okifuji et al. [47] | - | - | - | ** | ** | ** | * | 7 | |
| Ong et al. [44] | * | - | - | ** | ** | ** | * | 8 | |
| Parmelee et al. [35] | * | - | - | ** | ** | ** | * | 8 | |
| Robertson et al. [43] | - | - | - | * | ** | ** | * | 6 | |
| Segura-Jimenez et al. [42] | * | - | - | ** | ** | ** | * | 8 | |
| Tang et al. [31] | * | - | - | * | ** | ** | * | 7 | |
| Tang et al. [38] | * | - | - | * | ** | * | 5 | ||
| Whibley et al. [36] | * | - | - | ** | ** | ** | * | 8 | |
| Newcastle-Ottawa Scale for Cohort Studies | |||||||||
| Study | Selection | Comparability | Exposure | Total Score | |||||
| Representativeness of exposed cohort | Selection of nonexposed cohort | Ascertainment of exposure | Outcome not present at baseline | Assessment of outcome | Sufficient follow-up duration | Adequate follow-up | |||
| Blake et al. [18] | * | * | * | * | ** | * | * | * | 9 |
| Burgess et al. [25] | - | - | * | * | ** | * | * | * | 7 |
| Castano et al. [26] | * | - | * | * | ** | * | * | * | 8 |
| Gozani et al. [28] | * | - | * | * | ** | * | * | - | 7 |
The Utility of Actigraphy
We examined the utility of actigraphy to: 1) evaluate sleep after an intervention, 2) investigate the relationship between sleep and pain, 3) assess the relationship between sleep and physical activity, and 4) compare sleep in pain patients with healthy subjects.
Evaluating sleep after an intervention: The most common intervention examined was cognitive behavioral therapy (CBT) for insomnia, which was assessed in seven studies with 715 patients (Table 1) [18-24]. Actigraphy was able to detect postintervention improvements in various sleep parameters in six studies. Additionally, these studies showed improvements in subjective measures of sleep with CBT [18-23]. However, one study only showed improvements in subjective measures, while not finding any differences in actigraphic parameters between the CBT and control groups [24]. Two studies that also measured sleep with PSG found that the PSG findings agreed with the actigraphic findings [20,24]. Various other interventions were also assessed, and actigraphy was able to detect improvements in select sleep parameters [25-28].
Investigating the relationship between sleep and pain: Eight studies with 743 patients aimed to investigate the relationship between sleep, as measured with actigraphy, and pain (Table 1) [29-36]. While three studies found relationships between sleep and pain [29-31], five other studies did not [32-36]. Some of the studies that did not show relationships with actigraphic sleep parameters found relationships with subjective sleep quality [34,36].
Assessing the relationship between sleep and physical activity: Two studies with 151 patients examined the effect of sleep on physical activity and found that improved sleep led to greater physical activity levels during the day (Table 1) [37,38]. However, one of the studies only found the relationship with subjective sleep quality and not actigraphic sleep parameters [38]. Another study utilized actigraphy to examine the effect of physical activity on sleep, finding that greater fluctuations in daytime physical activity levels predicted reduced sleep duration and greater average daytime physical activity predicted greater time awake at night [39].
Comparing sleep in pain patients versus healthy subjects: Five studies with 356 patients used actigraphy to compare sleep in chronic pain patients versus healthy subjects [40-44], all studies except for one [44] found that sleep was worse in pain patients (Table 1). One study showed that chronic pain patients had greater time in bed than healthy subjects; however, sleep diary was also able to detect that chronic pain patients had higher SOL and WASO [40]. Fibromyalgia patients had delayed sleep onset, delayed waking, increased time in bed, and increased WASO compared to healthy subjects [41,42]. Chronic back pain patients had increased time in bed, TST, and SOL compared to healthy subjects; in contrast, PSG measurements did not detect a difference [43]. Interestingly, in a small study (n=40), chronic migraine patients did not have any differences in actigraphic sleep parameters compared to healthy subjects [44].
Meta-analysis
The concordance between objective and subjective measures of sleep was studied in five studies in fibromyalgia patients with mixed findings [42,45-47]. To further explore the concordance between different sleep assessment methods, meta-analyses were performed to compare actigraphy versus diary (Figure 2) and actigraphy versus PSG (Figure 3) for TST, SE, SOL, and WASO. The only significant difference was between actigraphy and diary in the measurement of SOL. Only four studies compared actigraphy with PSG whereas 12 studies compared actigraphy with sleep diaries.
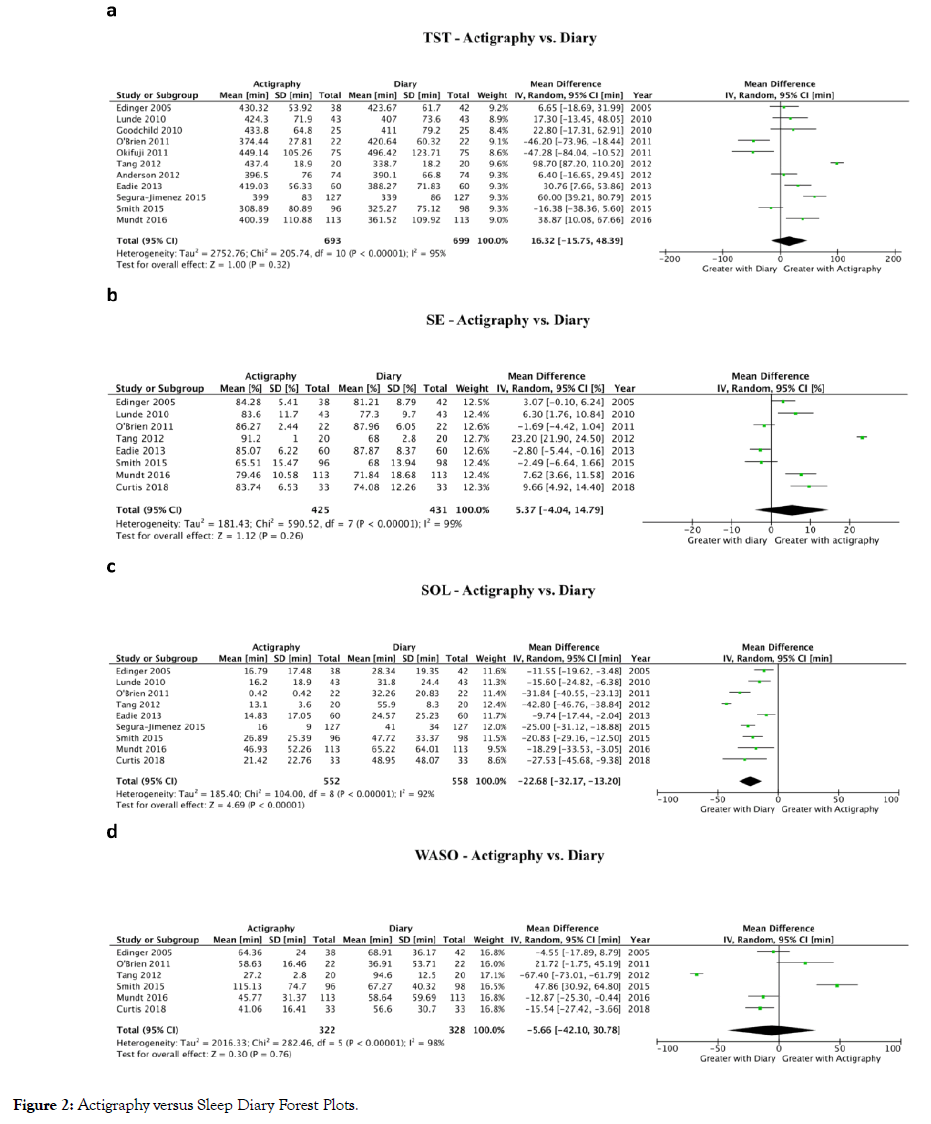
Figure 2: Actigraphy versus Sleep Diary Forest Plots.
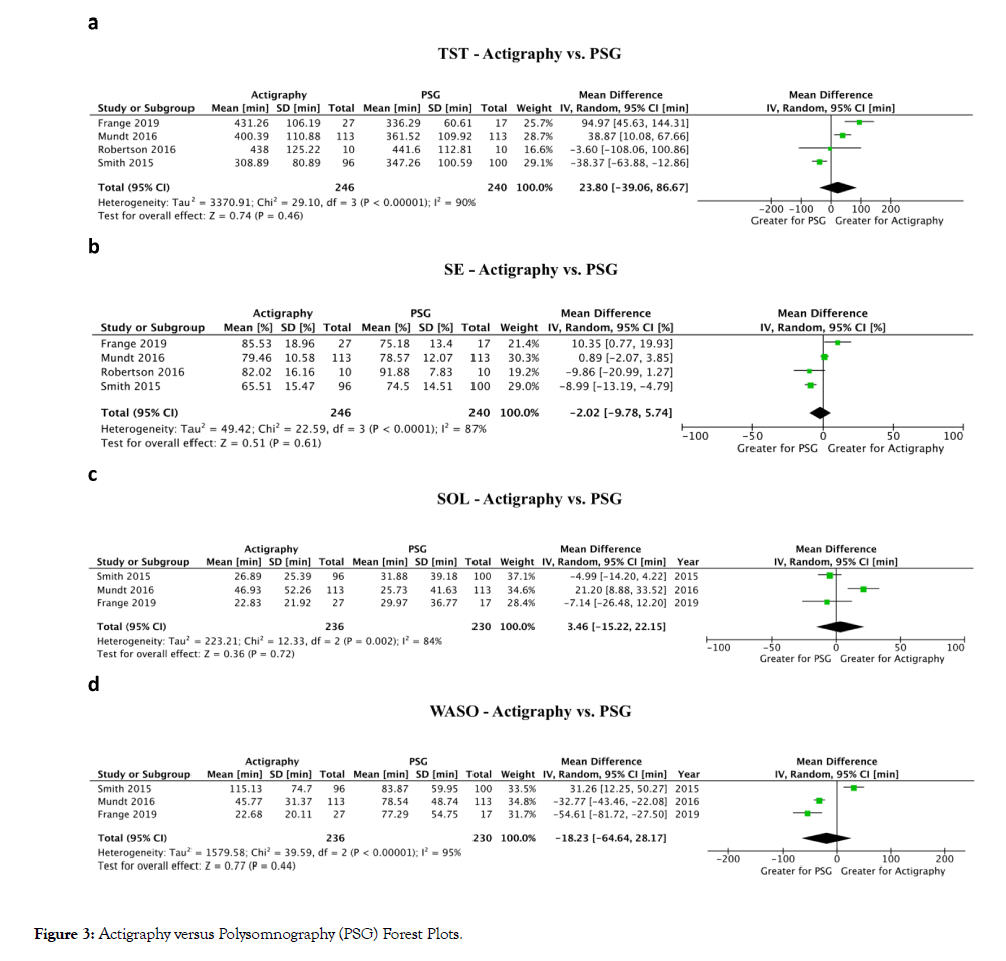
Figure 3: Actigraphy versus Polysomnography (PSG) Forest Plots.
Total sleep time (TST): Comparing actigraphy to diary (Figure 2a), a meta-analysis of 11 studies showed no difference in TST (16.3 min higher TST with actigraphy; 95% Confidence Interval (CI): 15.8 min lower to 48.4 min higher; P=0.32) [19,20,22,27,32,34,40,42,46,47,51]. Comparing actigraphy to PSG (Figure 3a), a meta-analysis of four studies showed no difference in TST (23.8 min higher TST with actigraphy; 95% CI: 39.1 min lower to 86.7 min higher; P=0.46) [20,29,43,46].
Sleep efficiency (SE): Comparing actigraphy to diary (Figure 2b), a meta-analysis of eight studies showed no difference in SE (5.4% higher SE with actigraphy; 95% CI: 4.0% lower to 14.8% higher; P=0.26) [19,20,22,27,34,40,46,49]. Comparing actigraphy to PSG (Figure 3b), a meta-analysis of four studies showed no difference in SE (2.0% lower SE with actigraphy; 95% CI: 9.8% lower to 5.7% higher; P=0.61) [20,29,43,46].
Sleep onset latency (SOL): Comparing actigraphy to diary (Figure 2c), a meta-analysis of nine studies showed that SOL was decreased with actigraphy (22.7 min lower SOL with actigraphy; 95% CI: 13.2 to 32.2 min lower; p<0.01) [19,20,22,27,34,40,42,46,49]. Comparing actigraphy to PSG (Figure 3c), a meta-analysis of three studies showed no difference in SOL (3.5 min higher SOL with actigraphy; 95% CI: 15.2 min lower to 22.2 min higher; P=0.72) [20,29,46].
Wake after sleep onset (WASO): Comparing actigraphy to diary (Figure 2d), a meta-analysis of six studies showed no difference in WASO (5.7 min lower WASO with actigraphy; 95% CI: 42.1 min lower to 30.8 min higher; P=0.76) [19,20,22,34,46,49]. Comparing actigraphy to PSG (Figure 3d), a meta-analysis of three studies showed no difference in WASO (18.2 min lower WASO with actigraphy; 95% CI: 64.6 min lower to 28.2 min higher; P=0.44) [20,29,46].
Feasibility of using actigraphy
One third of the 34 studies reported compliance with actigraphy. Compliance was high with six studies reporting >90% of participants completing actigraphy monitoring [25,29,31,36,38,47], and the other studies reporting between 42-77% of participants [27,28,35,37]. The rate of device malfunction was low, between 4% and 6.7% [25,27]. Regarding patient satisfaction, one study implemented a questionnaire and found that most patients were satisfied; 85% of participants said they would wear an actigraph again, and 78% said it was a userfriendly method of measuring sleep [27]. A second study also reported that patients found the actigraph easy to apply and use [18].
There is a growing interest in the use of actigraphy to measure sleep in chronic pain patients. This review aimed to provide an overview of the utility of actigraphy to measure sleep in chronic pain patients and to quantitatively summarize the concordance of actigraphy with sleep diary and PSG in this patient population. Actigraphy has been used to measure sleep in several contexts, providing a useful objective measurement; however, there are intrinsic differences between actigraphy and subjective measures as actigraphy lacks the cognitive component of subjective measures. Additionally, actigraphy estimates a significantly lower SOL compared to sleep diary. While no differences in any parameters were detected between actigraphy and PSG, the number of studies was limited and the variability was large.
Evaluating sleep after an intervention
After interventions designed to improve sleep, the postintervention improvement in actigraphic sleep parameters varied between studies [18-23]. In general, sleep diaries detected changes in more of the measured sleep parameters compared to actigraphy [19,22]. Additionally, one study found improvements with diary but not with actigraphy after the CBT intervention [24]. It appears that subjective measures of sleep are more “sensitive” than actigraphy. This is reasonable especially in the context of CBT for insomnia, which is a sleep disorder diagnosed based on self-reported symptoms [52]. Additionally, two studies showed that actigraphy agreed with PSG findings, providing some evidence that the two objective methods are consistent [20,24]. As such, actigraphy is able to provide an objective assessment along with other subjective measures when assessing sleep as an outcome of an intervention.
Investigating the relationship between sleep and pain
While some studies found an association between actigraphic sleep parameters and pain (29-31), other studies found no association [31-36]. Interestingly, the studies reporting an association included patients with comorbid insomnia, which could be a confounder. In studies that found no association between actigraphic sleep parameters and pain, there was often an association between diary sleep parameters and pain [34,36]. It appears that subjective measures of sleep are more “sensitive” to detect relationships between sleep and pain than actigraphy. Most prior studies that have found relationships between sleep and pain utilized subjective measures of sleep [2]. The degree of functional impairment caused by chronic pain for different individuals has an important cognitive component, comprised of pain-related beliefs and tendency to catastrophize [53]. There may be a cognitive component mediating the relationship that self-report measures are better able to detect. The utility of actigraphy to investigate the relationship between sleep and pain is inconclusive and should be further investigated. Investigating the relationship between sleep and physical activity
The major advantage of actigraphy is that it can objectively measure both sleep and physical activity levels. Physical activity is an important outcome to assess because it is effective at preventing and reducing chronic pain [54,55]. However, we found limited studies assessing the relationship between sleep and physical activity using actigraphy in chronic pain patients, necessitating future research in on this topic.
Comparing sleep in pain patients versus healthy subjects Consistent with prior studies, actigraphy also demonstrated that chronic pain patients had worse sleep compared to healthy subjects [2,56,57]. One small study (n=40) of chronic migraine patients did not find a difference in actigraphic sleep parameters between patients and healthy subjects. A previous study showed that migraine patients have worse subjective sleep quality [58]. Larger studies using actigraphy should be conducted to quantify sleep disturbances in chronic migraine patients. In general, actigraphy appears to be a useful tool to quantify sleep disturbances in chronic pain patients.
Concordance between actigraphy with diary and PSG
Comparing subjective measures of sleep versus actigraphy, SOL was significantly less with actigraphy while the other parameters were not significantly different. In chronic pain patients, insomnia is a common comorbidity [59,60]. Insomnia patients often report higher SOL than actigraphy; these patients possibly have a tendency to lie awake in bed without moving, which actigraphy detects as “sleep” [7,61]. Several studies in our metaanalysis also specifically recruited patients with comorbid insomnia [20,22,42,46,49]. As such, this could be a contributing factor to greater self-reported SOL amongst chronic pain patients. Previous studies have also reported that higher pain ratings in chronic pain patients are associated with longer subjective measures of SOL [62,63], suggesting a connection of subjective SOL with pain-related cognition.
Comparing PSG versus actigraphy, all four sleep parameters were comparable with no significant differences. Although not significant, TST was greater with actigraphy versus PSG and WASO was less with actigraphy versus PSG. The trend of actigraphy to overestimate “ sleep ” (TST) and underestimate “wake” (WASO) has been previously reported in a review that compared actigraphy versus PSG in patients with chronic conditions [12]. In patients with chronic pain or other chronic conditions, there could be changes to their mobility. This could alter the ability of actigraphy to accurately assess sleep because most actigraphy analysis software use algorithms that are only validated against healthy populations. One actigraph device, Actigraph-Insomnia, has a feature that aims to improve estimates of “wake” [38]. It involves a pressure sensor that patients hold between their finger and thumb, which is released when patients fall asleep and their muscle tone relaxes. The device could be tested in the future to see if it can improve the discrepancy. Based on thresholds set by the 2018 American Academy of Sleep Medicine on actigraphy [7], the 95% CI of the mean differences in our study were large and suggests that the two methods (actigraphy and PSG) cannot be used interchangeably. As such, even though no significant differences were found, it does not necessarily suggest that the two measurement methods are consistent and produce the same measurements.
This review has some limitations. With the restriction to English-language studies, we may not have identified all relevant literature. The included studies were heterogeneous with regards to patient populations and study designs. This variability likely led to the high statistical heterogeneity in the meta-analysis findings. Also, our meta-analyses comparing actigraphy with PSG contained few and small studies (total n=296). As well, five of the 12 studies comparing actigraphy with diary had less than 50 subjects.
Actigraphy is an objective assessment tool that is being increasingly utilized to measure sleep in chronic pain patients. As an adjunct to subjective measures like sleep diary, actigraphy provides a useful objective sleep metric when assessing the effect of an intervention and when comparing pain patients with healthy subjects; however, there are intrinsic differences between the assessment methods, and it is unclear which measure is more suitable for these uses. Diary measurements also tend to overestimate SOL compared to actigraphy. Even though no difference was detected between actigraphic and PSG parameters, it cannot be established that the two are equivalent measures due to limited studies and large variability in values. As actigraphy presents many potential advantages, further research is needed to compare the different assessment methods with large RCTs measuring sleep using multiple assessment methods in chronic pain patients.
DA devised the protocol, performed title/abstract and full-text screening, extracted outcomes, created Tables and Figures, and wrote and edited the manuscript. JS performed title/abstract and full-text screening, extracted outcomes, and wrote and edited the manuscript. JW wrote and edited the manuscript. CS wrote and edited the manuscript. SM wrote and edited the manuscript. ME conducted the literature search and edited the manuscript. FC devised the protocol, and wrote and edited the manuscript.
None
JW reports grants from the Ontario Ministry of Health and Long-Term Care, Anesthesia Patient Safety Foundation, Acacia Pharma, Merck Inc. outside of the submitted work. JW is supported by a Merit Research Award from the Department of Anesthesia, University of Toronto. FC reports research support from the Ontario Ministry of Health and Long-Term Care, University Health Network Foundation, Acacia Pharma, Medtronics grants to institution outside of the submitted work, and previous research grants from Pfizer. Up-to-date royalties, STOP-Bang proprietary to University Health Network. The other authors have no conflicts of interest to report.
Citation: An D, Selvanathan J, Wong J, Suen C, Mir S, Englesakis M, et al. (2020) The Utility of Actigraphy to Measure Sleep in Chronic Pain Patients and Its Concordance with Other Sleep Measures: A Systematic Review and Meta-Analysis. J Sleep Disord Ther 9:308.
Received: 27-Feb-2020 Accepted: 27-Mar-2020 Published: 03-Apr-2020 , DOI: 10.35248/2167-0277.19.9.308
Copyright: © 2020 An D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.