
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Review Article - (2024)Volume 15, Issue 6
Intraoperative Hypotension (IOH) is the most common cause of haemodynamic instability during general or spinal anesthesia. This phenomenon is associated with postoperative organ damage and death. The Hypotension Prediction Index (HPI) is the first machine learning software that may predict the appearance of hypotension, defined as a decrease in mean arterial pressure below 65 mmHg, at least 5 min in advance. This software is based on subtle arterial waveform changes induced by hypotension since its early stages. The present review analyses prospective as well as retrospective clinical published studies on the validation and reduction of IOH using HPI software during the last 6 years. Current evidence supports that HPI predicts with high accuracy the onset of hypotension and almost all evidence shows a significant reduction due to its use. However, the data concerning whether HPI may also improve postoperative outcomes is currently scarce and inconsistent.
Hypotension; Anesthesia; Hypotension prediction index; Haemodynamic monitoring
Intraoperative Hypotension (IOH) is the most common cause of haemodynamic instability in perioperative settings. Hypotension during surgery has been associated with vital organ damage and death, in large retrospective or prospective studies [1-5]. Thus, it is plausible to believe that the avoidance of IOH will reduce organ injury and improve postoperative outcomes. However, at present, no one has definitively demonstrated that this hypothesis may be true [6].
Hatib et al., were the first to develop a software based on machine learning technology to predict real-time IOH; the Hypotension Prediction Index (HPI) [7]. Since Hatib et al., publication, the number of research articles using HPI software to avoid hypotension, defined as a decrease in Mean Arterial Pressure (MAP) below 65 mmHg, has grown with time [8-15].
The aim of the present review is to summarize the literature concerning the prediction/reduction of IOH in HPI literature published after more than 5 years of its clinical use [16-21]. We will also discuss the studies validating the algorithm as well as recent criticism that surrounds the HPI software statistical performance. We will provide data that may help to clarify this question.
The present review includes all publications registered on the PubMed database web from the National Center for Biotechnology Information (NCBI). The words used for the search were “Hypotension Prediction Index and Hypotension”, which includes all studies published between Hatib et al., publication, 2018 and March 2024 and restricted to the English language [7].
The hypotension issue
After all these years, there is still controversy about what we mean by the term hypotension. The IOH definition changes according to physicians’ criteria. Most make reference to absolute thresholds, while others focus on relative changes of MAP or Systolic Arterial Pressure (SAP) [22]. Currently, the definition of hypotension is unclear regardless of the type of anesthesia used [22,23]. However, this is not a minor issue, since IOH has been associated with the occurrence of postoperative major organ injury and death [1-4]. The depth or severity, as well as the time of exposure to IOH, at any given threshold, determines the postoperative damage [24]. To clarify this question, the perioperative quality initiative group reached a consensus definition of hypotension, which concluded even brief periods of intraoperative values of MAP below 60-70 mmHg and/or SAP below 100 mmHg are associated with major organ damage; hence they should be avoided [25]. The etiology of IOH is multifactorial and could be simplified into three main factors: i) Hypovolemia (intraoperative bleeding), ii) vasodilatation and iii) myocardial depression (most frequently induced by general anesthetics) [25]. IOH treatment is another focus of discussion. Ideally, it should be directed to abort its real cause, although, in most of the cases, it is reactive and empirical.
The next issue to address is how we measure hypotension. Time or duration of IOH during a procedure alone is not enough, since it does not account for its severity. The Time Weighted Average (TWA) is a concept developed to measure IOH that includes both dimensions [26]. TWA is measured by calculating the Area Under a determined Threshold (AUT), for instance, MAP<65 mmHg, divided by the duration of a surgical procedure, that is, TWA= (depth of hypotension in mmHg of MAP<65 mmHg × time in min spent below that threshold)/Total duration of surgery in minutes [9,26]. The advantage of using the TWA is that it allows a comparison of the severity of IOH between institutions and publications (Figure 1).
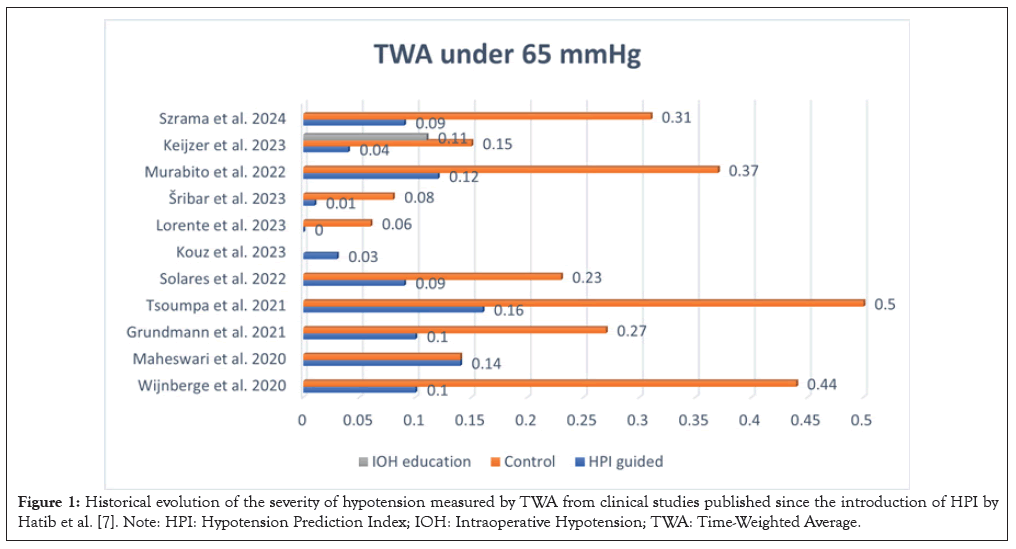
Figure 1: Historical evolution of the severity of hypotension measured by TWA from clinical studies published since the introduction of HPI by Hatib et al. [7]. Note: HPI: Hypotension Prediction Index; IOH: Intraoperative Hypotension; TWA: Time-Weighted Average.
Development of the HPI Algorithm
To identify incoming hypotensive events, Hatib et al., constructed the HPI algorithm [7]. This author defined IOH using a threshold of a decrease in MAP below 65 mmHg [7,23]. Specifically, Hatib et al., to statistically analyses this prediction, labelled hypotension as MAP<65 mmHg and norm tension as MAP>75 mmHg, demarcating a Grey zone for which data was not analyzed [7]. The HPI value is displayed on a monitor as a number ranging from 1 to 100, where the first warning of the appearance of an IOH event occurs when the value exceeds 85.
Whenever an organism faces a challenge, the induced haemodynamic instability produces subtle yet complex changes in various cardiovascular variables from the early stages [27,28]. These dynamic changes present in the arterial pressure waveform can only be detected by machine learning methods [7]. The HPI algorithm was developed using powerful mathematical tools that quantified these compensatory cardiac and baroreceptor mechanism changes before a hypotensive event appeared. The predictor underwent an internal and external validation with a cohort of 350 intensive care patients and 204 surgical patients, respectively. This led to a high prediction accuracy rate and an Area Under the receiver operating Curve (AUC) of 0.95, 0.95 and 0.97 for 15, 10 and 5 min before the event, respectively [7].
However, Enevoldsen et al., have recently criticized the methodology used to perform the prediction [29]. They speculated that the selection bias of the Grey zone, forces classification as hypotension the events with MAP below 75 mmHg, which creates a biased model that overestimates the risk of hypotension [29]. In their opinion, the model will be an overrepresentation of the changes in MAP that overcome those of the waveform features. Thereafter, Mulder et al., published a research letter showing data from 18 surgical patients who developed hypotension (MAP<65 mmHg) [30]. They found a highly negative correlation between the trend values of MAP and HPI [30]. The authors agree with Enevoldsen et al., findings and hypothesized that HPI is not different than other predictors based on MAP trend values [29,30]. Thus, these papers have introduced doubts and opened a debate about whether HPI is based on baroreceptor induced changes or simply follows a MAP trend. Unfortunately, the current literature on the topic of this controversy, at least at the clinical level, is scarce or non-existent [6].
In 2018, we ran a prospective observational pilot trial of 48 adult patients that received Spinal Anesthesia (SA). We tested if HPI>85 warning values could predict the treatment of hypotension using two different definitions of hypotension; one was based on SAP values below 100 mmHg (25, American Society of Anesthesiologists (ASA) III-IV patients) and the other SAP values below 90 mmHg (23, ASA I-II patients). Bradycardia was treated when Heart Rate (HR) was ≤ 51 beats per minute (bpm) in both groups. Blood pressure was non-invasively monitored with a clear sight finger cuff connected to an EV1000NI monitor. Since HPI technology was not available on clear sight at that time, HPI values were calculated posteriori by engineers of Edwards Life Sciences (Irvine, CA). So, the anesthesiologists were blinded as regards HPI values at Treatment Time (TT). Interestingly, 9 of 48 (18.7%) patients in the study were treated due to bradycardia. After hypotension, bradycardia is the second most common cause of haemodynamic instability due to SA [31,32]. Moderate bradycardia, HR below 50 bpm, appears in 10-13% of cases and if untreated, it could lead to cardiovascular collapse and/or asystole [31,32]. Unlike hypotension, the pathophysiology of this bradycardia is not well understood [32]. The blockade of cardiac accelerator fibers of the heart when sympathetic blockade reaches T4-T8 levels is one theory. In this case, bradycardia should appear without a concomitant decrease in blood pressure. The other two involve the cardiopulmonary baroreceptors located in the right atria, superior and inferior vena cava, and pulmonary circulation [32]. As well as the ventricular mechanoreceptor located in the ventricular wall of the heart, the Bezold-Jarisch reflex. In either of both cases, the stimulation of one of these structures results in the inhibition of the tonic discharge of the sympathetic system while, in opposition, excites the vagal innervation of the heart. The result is bradycardia, vasodilatation, a drop in blood pressure and a decrease in cardiac output. In 5 patients out of 9 of our study, the HPI>85 warning values predicted bradycardia treatment at least 5 mins before (Table 1). In the other 4, this did not happen in any patient at any time (Table 2). The haemodynamic behaviour was totally different between both groups. In the latter, the trend to moderate bradycardia 10 mins before was the main change (Table 2). In the former, the decrease in HR was accompanied by a response compatible with an inhibition of vasoconstrictor innervation. This data suggests that HPI warning values could only predict bradycardia treatment caused by an inhibition of the baroreceptor-mediated sympathetic system at its origin. The fact that atropine administration restored cardiovascular performance values confirms such an assumption (Table 1). This clinical evidence is in agreement with Longrois et al., who have recently stated that HPI is based on a strong physiologic/pathophysiologic basis, that is, the cardiopulmonary baroreflexes [6]. They have pointed out that the arguments used to open this HPI controversy are more related to the statistical methodology of estimating blood pressure measurements [6]. According to Longrois et al., Hatib et al., could do better in their chosen definition of hypotension as the Grey zone, opening a door to improve the statistical analysis of HPI in the future [6,7]. In the meantime, we suggest continuing to work with the system and letting the statisticians resolve their debate.
| n=5 | BASAL | 10BTT | 5BTT | TT | 5ATT |
|---|---|---|---|---|---|
| SAP | 132 ± 27 | 120 ± 21 | 104 ± 7 | 101 ± 14 | 117 ± 15 |
| MAP | 95 ± 14 | 85 ± 24 | 71 ± 3 | 70 ± 5 | 84 ± 11 |
| HR | 72 ± 8 | 59 ± 6 | 55 ± 4 | 49 ± 3 | 72 ± 17 |
| CI | 3.1 ± 0.6 | 2.6 ± 0.8 | 2.5 ± 0.7 | 2.3 ± 0.7 | 3.4 ± 1.1 |
| SVI | 39 ± 12 | 46 ± 16 | 45 ± 14 | 47 ± 14 | 40 ± 13 |
| SVRI | 2766 ± 992 | 2040 ± 771 | 2242 ± 671 | 2334 ± 669 | 2529 ± 575 |
| dp/dt | 610 ± 210 | 548 ± 142 | 493 ± 163 | 460 ± 173 | 550 ± 203 |
| HPI | 18 ± 18 | 57 ± 38 | 93 ± 3 | 94 ± 4 | 49 ± 30 |
| AUC | 0.733 | 1.0 | 1.0 |
Note: 10BTT: 10 Minutes Before Treatment; 5ATT: 5 Minutes After Treatment; 5BTT: 5 Minutes Before Treatment; AUC: Area Under the receiver operating Curve; CI: Cardiac Index; HPI: Hypotension Prediction Index; HR: Heart Rate; MAP: Mean Arterial Pressure; SAP: Systolic Arterial Pressure; SVI: Stroke Volume Index; SVRI: Systemic Vascular Resistance Index; TT: Treatment Time.
Table 1: Main haemodynamic data before and after treatment of atropine in 5 patients to treat moderate to severe bradycardia that was predicted by HPI during spinal anesthesia.
| n=4 | BASAL | 10BTT | 5BTT | TT | 5ATT |
|---|---|---|---|---|---|
| SAP | 136 ± 24 | 130 ± 25 | 125 ± 23 | 132 ± 17 | 139 ± 11 |
| MAP | 94 ± 14 | 86 ± 10 | 85 ± 11 | 88 ± 6 | 97 ± 5 |
| HR | 61 ± 7 | 55 ± 8 | 52 ± 3 | 47 ± 1 | 72 ± 16 |
| CI | 2.5 ± 0,5 | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.5 ± 0.6 | 2.7 ± 0.7 |
| SVI | 41 ± 8 | 43 ± 4 | 43 ± 3 | 46 ± 4 | 37 ± 3 |
| SVRI | 2945 ± 666 | 2934 ± 417 | 2902 ± 355 | 3075 ± 461 | 2897 ± 697 |
| dp/dt | 665 ± 203 | 623 ± 204 | 610 ± 278 | 600 ± 205 | 686 ± 227 |
| HPI | 22 ± 16 | 30 ± 19 | 37 ± 25 | 25 ± 10 | 13 ± 5 |
| AUC | 0.25 | 0.0 | 0.0 |
Note: 10BTT: 10 Minutes Before Treatment; 5ATT: 5 Minutes After Treatment; 5BTT: 5 Minutes Before Treatment; AUC: Area Under the receiver operating Curve; CI: Cardiac Index; HPI: Hypotension Prediction Index; HR: Heart Rate; MAP: Mean Arterial Pressure; SAP: Systolic Arterial Pressure; SVI: Stroke Volume Index; SVRI: Systemic Vascular Resistance Index; TT: Treatment Time.
Table 2: Main haemodynamic data before and after treatment of atropine in 4 patients to treat moderate to severe bradycardia that was not predicted by HPI during spinal anesthesia.
Clinical validation of the HPI system
Davies et al., published the first large clinical validation trial of the HPI algorithm in 2020 [33]. In 255 non-cardiac surgical patients receiving Goal Directed Fluid Therapy (GDFT) optimization and with an arterial line, they found a high prediction of the onset of IOH at 5, 10 and 15 mins before the event with an AUC of 0.926, 0.895 and 0.879, respectively. Interestingly, this prediction had a sensitivity and specificity above, 85% 5 mins before the event [33]. However, Ranucci et al., studied the ability of the algorithm to predict IOH in 23 patients, 20 of them during cardiac surgery [34]. These authors selected HPI values, although avoiding those from the bias zone and found a prediction of an AUC of 0.768 at 5-7 mins beforehand. Thereafter, Shin et al., reported in the same cardiac surgical setting with 37 participants a prediction of IOH at 5 mins of an AUC of 0.90 [35].
The next question will be if HPI could have the same prediction rate with non-invasive continuous devices, during SA with spontaneous breathing patients and/or other thresholds or definitions of IOH. The answer to this question is yes. An observational cohort study with 507 adult patients undergoing general surgery studied the performance of the HPI algorithm using a Non Invasive Blood Pressure finger cuff (NIBP) in 404 patients versus invasive arterial blood pressure measurements in the remaining 103 [36]. The performance of the algorithm with NIBP resulted in an AUC of 0.93, 0.91 and 0.90 at 5, 10 and 15 min respectively prior to the hypotensive event, similar to invasive results. Maheswari et al., also found similar prediction rates in the same periods with an NIBP device in 320 ASA status III-IV non-cardiac surgery individuals under general anesthesia and HPI values between 80-89 provided a median of 6 mins warning time before IOH appeared [37]. On the other hand, Frassanito et al., tested the performance of the HPI with an NIBP in 50 awake Cesarean Delivery (CD) patients under SA. In this retrospective analysis, the HPI predicted maternal hypotension 3 mins before, with an AUC of 0.913 [38]. Indeed, in our prospective blinded study in 24 SA non-CD patients, we found a good prediction rate of 0.883 of warning of treatment with HPI>85 values at 3 mins before hypotension defined as a decrease in SAP<100 mmHg and of 0.847 with a definition of SAP<90 mmHg.
Clinical use and hypotension reduction with the HPI-Algorithm
To date, there are 14, 10 prospective and 4 retrospectives, observational clinical studies on hypotension reduction that met the requirements for clinical use of the HPI algorithm and one that did not [8-15,18]. In these studies, the presence of IOH was measured using the TWA in most of them (11 out of 14), while in the others, by the incidence of perioperative hypotensive episodes or by the time of their cumulative duration [16-21]. Of these 14, all showed a significant reduction in hypotension in patients guided by HPI compared to those managed under GDFT or institutional protocols, except by two (Table 3 summarizes all of the data). One of these two did not have a control group and therefore, no differences could be established [15]. The other was a randomized pilot study of 214 non-cardiac surgical patients from Maheshwari et al., in which 105 patients were managed with a complex HPI-guided protocol compared with another group of 108 that received a conventional one and showed a similar hypotension rate in both groups of a TWA of 0.14 [10]. However, the authors admitted that their protocol was complex and the clinicians involved were apprehensive about the use of an unfamiliar technology. Thus, practically half of the alarms were not followed by treatment, leading to an inappropriate use of the system, which may explain these conflicting results [10]. This suggests that specific education and training of physicians on new technology could be a key determinant in the success of the clinical use of HPI [14,19]. In this respect, Keijzer et al., have recently shown in a smart study how specific education of clinicians in HPI software use could affect the results of IOH treatment [19]. 25 patients of the study received an institutional GDFT protocol and standard monitoring to manage their IOH; this was defined as a baseline cohort group. Subsequently, 25 new patients were managed by the same group of anesthesiologists, although instructed on the risks and adverse outcomes of IOH and specifically avoiding MAP<65 mmHg values; this was the educational cohort. Finally, other 25 new patients were managed by the same clinicians, although specifically trained in HPI technology to keep MAP>65 mmHg; this was the HPI cohort. They found that the HPI cohort group showed significantly lower IOH, with a TWA of 0.04, compared with the baseline cohort with a TWA of 0.15 (p<0.05) or the educational cohort with a TWA of 0.11 (p<0.05) [19].
| Design | Patients | Primary Outcome | Secondary Outcome | Estimated Blood loss | Cardiac output target | p value | Results |
|---|---|---|---|---|---|---|---|
| Single centre randomised blinded prospective trial (Shneck E, ref 8) |
HPI guided (n=25), Control (n=25), Historic control (n=25). Total hip arthroplasty under general anaesthesia. | Perioperative incidence of hypotensive episodes. | Absolute and relative duration of IOH events. Fluid therapy, vasoactive drugs. | HPI 700 ml, Ctrl 550 ml, hCTRL 600 ml | CI ≤ 2.0 l/min/ m² *Individual CI: HPI>80, SVV<12% and decreased CI (compared to baseline before induction) | 0.001 | Hypotensive events per hour. HPI guided 0 (0-1), Control 5 (2-6), Historic control 2 (1-3) Significant reduction in IOH in the HPI group compared with the control groups (HPI 48%, CTRL 87.5%, hCTRL 80%) Algorithm compliance of 77.8% based on 119 HPI triggered interventions, 26 protocol violations and an overall amount of 153 therapeutic actions. |
| Unblinded randomised clinical trial (Wijnberge M, ref 9) |
Early warning system (n=34) or standard care (n=34). Elective non-cardiac surgery | The primary outcome was Time-Weighted Average of hypotension during surgery (TWA) | Incidence, total time with hypotension and percentage of time spent with hypotension during surgery. | No data | No data | 0.001 | Intervention group median TWA 0.10 mmHg vs. 0.44 mmHg in the control group. Incidence, number of events, 3.0 (1.0-8.0) vs. 8.0 (3.5-12.0). Total time under 65 mmHg, min 8.0 (1.3-26.0) 32.7 (11.5-59.7). |
| Randomised Clinical Trial (RCT) (Maheshwari K, ref 10) |
HPI guided (n=105) Unguided (n=108) Moderate high risk, non-cardiac surgery. | TWA of IOH under 65 mmHg | Secondary outcomes were time-weighted mean pressures less than 60 and 55 mmHg. | HPI 200 ml CTRL 200 ml | No data | p=0.75 | No significant differences. HPI guided median TWA 0.14 mmHg vs. 0.14 mmHg in the control group. TWA 0.28 mmHg historic control. |
| Retrospective observational (Grundmann CD, ref 13) |
HPI (n=50) Flotrac (n=50) Moderate- or high-risk non-cardiac surgery | TWA of IOH under 65 mmHg | Number of patients with hypotensive events, number of events per patient, cumulative and average duration of hypotension. | HPI 550 ml Flotrac 450 ml | No data | p=0.001 | Median time-weighted average of hypotension 0.10 (0.19) mmHg in the HPI group vs. FloTrac group 0.27 mmHg. |
| Prospective RCT (Tsoumpa M, ref 11) | HPI n=49, non-HPI n=50 moderate- or high-risk non-cardiac surgery | TWA of IOH under 65 mmHg | Time spent in hypertension defined as Mean Arterial Pressure (MAP)>100 mmHg for at least 1 min; medication and fluids administered and postoperative complications. | HPI 350 ml, Control 500 ml | No data | p=0.0003 | Median TWA of hypotension was 0.16 mmHg in the intervention group versus 0.50 mmHg in the control group. TWA hypertension, HPI guided, mmHg 0.95 vs. 0.29 non HPI. Number of hypertensive events, n 73 HPI guided vs. 34 control. Time in Hypertension, HPI min 24.30 vs. 11.33 control. There were no differences in adverse events. |
| Retrospective observational (Solares GJ, ref 14) |
104 (HPI n=52, GDFT n=52) Urgent or elective non-cardiac surgery with moderate-to-high risk of bleeding | TWA of IOH under 65 mmHg | Postoperative complications and length of hospital stay. | GDFT 661 ml, HPI 607 ml | CI ≤ 2.0 l/min/ m² | p=0.037 | Median TWA of IOH in the HPI group 0.09 vs. 0.23 in the GDFT. LOS was significantly shorter in the HPI group, with a median difference of 2 days (p=0.019). |
| Multicentre registry (Kouz K, ref 15) | n=702 elective major non-cardiac surgery expected duration >120 min | TWA of IOH under 65 mmHg | The proportion of patients with at least one >1 min episode of MAP <65 mmHg and duration patients spent below a MAP of 65 mmHg. | 250 ml | No data | No comparison group | TWA under 65 mmHg 0.03 absolute duration below MAP threshold 65 mmHg 2 min. Proportion of patients with at least one >1 min episode below MAP thresholds <65 mmHg 59.4%. |
| Multicenter RCT (Llorente JV, ref 16) | HPI guided n=40 Goal-Directed Hemodynamic Therapy n=40 Major abdominal surgery. | TWA of IOH under 65 mmHg | Intraoperative Skeletal muscle tissue Oxygenation(StO2). Lactate levels. Acute Kidney Injury (AKI) Risk. Urinary Tissue Inhibitor of Metalloproteinase (TIMP-2)- Insulin-Like Growth Factor Binding Protein (IGFBP7). Postoperative complications and length of stay. | No data | GDHT, an inotropic agent, was added if the CI persisted at<2.5 l/min/m² in patients with SVV<13% MAP<65 mmHg | p=0.015 | Median TWA HPI guided was 0 vs. 0.06 in the GDHT group. For secondary endpoints there were no statistically significant differences. |
| Prospective, randomised, single-blinded study (Sribar A, ref 17) | Elective major thoracic procedure. 34 patients n=17 acumen IQ, n=17 FloTrac | TWA of IOH under 65 mmHg | Anesthesia and Ultrasound Technology (AUT), number of hypotensive episodes, cumulative duration of hypotension | No data | CI ≥ 2.4 l/min/m² | p=0.04 | Acumen guided group TWA 0.01 vs. 0.08 FloTrac guided group. Hypotensive events 0 vs. 2. Duration of hypotension 0 min vs. 3.7 min. |
| Prospective, single-arm trial, compared to a historical comparison cohort. (Bao X, ref 21) |
Trial subjects (n=406) and 15,796 comparison patients. 18 years or older, ASA physical status 3 or 4 and scheduled for moderate- to high-risk non-cardiac surgery expected to last at least 3 h. | Cumulative duration of intraoperative hypotension | Area under Mean Arterial Pressure (MAP) of 65 mmHg | No data | No data | p<0.001 | Acumen guided, 9 ± 13 min of MAP below 65 mmHg, compared with the historical control mean of 25 ± 41 min, a 65% reduction |
| Single-centre pilot randomised clinical trial. Intervention group, n=20; standard care group, n=20. (Murabito P, ref 12) |
≥ 18 years old undergoing elective laparotomic major general surgery under general anaesthesia. | TWA, difference in hypotension (defined as mean arterial pressure<65 mmHg) | Surrogate markers of organ injury and oxidative stress. Biomarkers of major organs, including the brain (Neuron-Specific Enolase (NSE) and S100B protein), heart (high-sensitive Troponin (hsTPN) and kidney (Neutrophil Gelatinase-Associated Lipocalin (NGAL) were assessed in all patients. | No data | No data | p=0.048 | The median time-weighted average of hypotension was 0.12 mmHg (0.35) in the intervention group and 0.37 mmHg (1.11) in the control group. NGAL correlated with TWA of hypotension (R=0.32; p=0.038) and S100B with number of hypotensive episodes, absolute time of hypotension, relative time of hypotension and time-weighted average of hypotension (p<0.001 for all). The intervention group showed lower NSE and higher reduced glutathione when compared to the control group. |
| Single-centre retrospective study (Szrama J, ref 20) |
Moderate and high-risk patients undergoing major abdominal surgery. All patients, n=123; FloTrac, n=61; HPI, n=62 | Time-Weighted Average (TWA) of hypotension below<65 mmHg | Average number of hypotensive events for MAP<65 mmHg and<50 mmHg. Cumulative and average duration of hypotensive events<65 mm <50 mmHg, the number of patients with hypotensive events<65 mmHg and<50 mmHg and TWA for MAP<50 mmHg. | 150 ml FloTrac group, 150 ml HPI group. | No data | p=0.000009 | TWA of hypotension in the FloTrac group was 0.31 mmHg versus 0.09 mmHg in the HPI group. In the FloTrac group, the average time of hypotension was 27.9 min vs. 8.1 min in the HPI group. |
Note: IOH: Intraoperative Hypotension; CTRL: Control; hCTRL: historic Contol; HPI: Hypotension Prediction Index; NGAL: Neutrophil Gelatinase-Associated Lipocalin; TWA: Time Weighted Average: Time weighted Average; NSE : Neuron-Specific Enolase; ASA: American Society of Anesthesiologists; GDHT: Goal-Directed Hemodynamic Therapy; hsTPN: heart high-sensitive Troponin; AUT: Anesthesia and Ultrasound Technology; MAP: Mean Arterial Pressure; AKI: Acute Kidney Injury; TIMP: Tissue Inhibitor of Metalloproteinase; IGFBP: Insulin-Like Growth Factor Binding Protein; SVV: Stroke Volume Variation.
Table 3: Summary of the most significant data from the clinical studies that used HPI.
Along with others, we also believe that it is as important for an algorithm to be a good predictor of hypotension as it is to provide us with the tools for its correct diagnosis and, therefore, a better treatment [6,14]. In our view, the HPI meets both conditions and this is one of the greatest advances made by the system. The HPI technology allows the clinician to monitor known advanced physiological variables, such as Stroke Volume Variation (SVV) and innovative, less frequent ones, such as dynamic arterial elastance (Eadyn) and dP/dtmax. Using such monitoring, we were able to manage the haemodynamic instability after anesthesia induction in a patient with a moderate dilated cardiomyopathy without the use of a more invasive Swan-Ganz catheterization [39]. Moreover, we believe that protocols based on the secondary screen of HPI software directed to detect the pathophysiology of arterial hypotension, that is hypovolemia, vasoplegia and myocardial depression, are the best choice [9,14]. Although, we have to admit that other alternatives have reached similar results [13]. However, we have to keep in mind that the maintenance of both perfusion pressure and cardiac output are essential goals to achieve adequate support for organ metabolism [14,21]. We must not make the mistake of looking only at the pressure and forgetting about the flow. Patients should be normovolemic at the same time that they are free of IOH.
Outcome and HPI algorithm use
The last question of this review is whether the reduction of IOH with this novel technology has resulted in a lower incidence of postoperative complications. However, the literature covering this topic is very limited. For instance, no statistically significant difference was observed in acute kidney injury in different studies despite a reduction of IOH with the algorithm [16,21]. On the other hand, Reddy et al., observed a shorter ventilation time in those Intensive Care Unit (ICU) patients in whom the HPI was used [40]. Murabito et al., have reported a significant reduction in the IOH end-organ injury biomarkers and oxidative stress with the intervention of HPI [12]. We have found a lower postoperative complication rate per patient and a shorter length of hospital stay, a median difference of two days in the HPI group, in our observational retrospective study [14]. More recently Andrzejewska et al., also reported in a prospective study of patients undergoing posterior spinal fusion for idiopathic scoliosis a significant reduction in hospital stay, a median difference of three days, in the intervention HPI group [18]. Unfortunately, the mean age of these patients was 15 years old and it is known that HPI software is not validated for patients with an age below 18 years [7].
IOH remains a significant cause of hemodynamic instability during surgery, linked to organ damage and adverse outcomes, though a definitive causal relationship between its prevention and improved postoperative results has yet to be established. One important tool for managing IOH is the HPI, a machine learning-based algorithm developed by Hatib et al., to predict the onset of hypotension (MAP <65 mmHg) in real time. The HPI has been validated in several studies, demonstrating high prediction accuracy and early precautions, but its statistical methodology and potential biases have been attacked. Analysts argue that HPI may rely too heavily on MAP trends rather than truly detecting baroreceptor-induced physiological changes. Despite these concerns, clinical studies have generally shown that HPI-guided management can reduce IOH, though outcomes such as postoperative complications and length of hospital stay have shown mixed results. Training and proper implementation of the technology appear important for maximizing its benefits. While more research is needed, the HPI offers an important tool for improving intraoperative hemodynamic management and potentially reducing organ injury.
There is enough evidence to support the contention that HPI software predicts IOH, defined as a decrease in MAP below 65 mmHg, at least 3 to 5 mins in advance. Independently of the protocol used, there is enough evidence to conclude that HPI significantly reduced IOH. Moreover, HPI incorporates software that helps us identify its cause with greater certainty. However, the existing literature regarding whether this reduction improves postoperative outcomes is currently inconclusive. Future prospective randomized and multicenter clinical trials are needed in order to determine if the use of HPI is able to reduce postoperative organ damage. The HPI is the first method based on machine learning technology in anesthesia monitoring. We are at the beginning and there is little doubt that artificial intelligence is the future in our clinical practice.
Gumersindo Solares has received in the past fees for lectures from Edwards Lifesciences Ltd. Currently this author has no relationship with Edwards Lifesciences Ltd., or any company of the anesthesia monitoring field. Daniel Garcia received fees for lectures from Edwards Lifesciences Ltd.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Solares G, Garcia D (2024). The Value of the Hypotension Prediction Index as a Tool for Treatment of Haemodynamic Instability: A Clinical Review. J Anesth Clin Res. 15:1164.
Received: 06-Nov-2024, Manuscript No. JACR-24-34996; Editor assigned: 11-Nov-2024, Pre QC No. JACR-24-34996 (PQ); Reviewed: 27-Nov-2024, QC No. JACR-24-34996; Revised: 05-Dec-2024, Manuscript No. JACR-24-34996 (R); Published: 30-Dec-2024 , DOI: 10.35248/2155-6148.24.15.1164
Copyright: © 2024 Solares G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.