Gynecology & Obstetrics
Open Access
ISSN: 2161-0932
ISSN: 2161-0932
Research Article - (2019)Volume 9, Issue 9
Background: Ovarian torsion is a serious gynecological condition, encountered especially during reproductive age. Medical ozone has therapeutic effects with its antioxidant, apoptotic, and anti-inflammatory properties.
Objective: This study aimed to determine the effects of medical ozone on the ovarian functions and ovarian morphology in an experimental rat ovarian torsion-detorsion model.
Methods: Twenty female Wistar albino rats were used in this study. The rats were randomized into two groups: Group 1 (torsion/detorsion+ozone) (n=10) and Group 2 (only torsion/detorsion) (n=10). Histopathological evaluation of the ovarian tissue was performed. Additionally, Total Antioxidant Capacity (TAC), Follicle-Stimulating Hormone (FSH), Estradiol (E2), and Lactate Dehydrogenase (LDH) levels were measured in the serum.
Results: Ozone application has to lead to a decrease in histopathological parameters such as hemorrhage, vascular congestion, cellular apoptosis, and necrosis while no histopathological changes were detected concerning edema and inflammatory cells. High TAC in Group 1 and high FSH in Group 2 were detected. A statistically significant difference between the two groups was detected in terms of TAC (p=0.001) and FSH (p=0.002). There were no significant differences between Group1 and Group2 regarding E2 (p=0.757), and LDH levels (p=0.453).
Conclusion: edical ozone is shown to positively affect the histopathological markers of cellular damage and increase the antioxidant capacity in the torsion-detorsion model. We suggest that further animal model studies may be designed to reveal the factors behind the effect of medical ozone.
Ischemia; Reperfusion; Ozone; Ovary; Torsion
Ovarian torsion (adnexal torsion), generally occurs in the presence of a big ovarian and/or para tubal cysts. It is a serious gynecological condition seen in women, especially in their reproductive age (15 to 49 years) with a prevalence of 2-3% all over the world [1-3]. Ovarian torsion leads to an interruption in blood flow of ovarian tissue. This torsion process, in turn, leads to an ischemic injury presented by ovarian necrosis, follicular degeneration, infarction, vascular congestion, bleeding, edema, and inflammatory cell infiltration [1]. Early diagnosis and treatment may prevent fertility decline and ovary loss. However, clinical findings and non-specific symptoms may significantly delay the diagnosis [4,5].
The treatment of ovarian torsion involves oophorectomy, conservative approaches involve detorsion of the twisted-ovary. At that point, detorsion may have local and systemic outcomes depending on the reperfusion process [6]. During detorsion, ovarian reperfusion leads to a “reperfusion injury” by the activation of thrombocytes and neutrophils, the secretion of proinflammatory agents, the formation of free radicals, and the production of large amounts of Reactive Oxygen Species (ROS) [7]. This pathological process is known as the “ischemiareperfusion (I/R) injury” or “ischemia-reperfusion damage.”
I/R injury is known as a major cause of ovarian damage caused by adnexal torsion/detorsion and is usually seen with the inflammation and oxidative stress, which is occurring by an imbalance between ROS and the anti-oxidative defense system [3,8]. Extended production of ROS breaks the nucleic acid chain and leads to the denaturation of proteins including some enzymes [2,9].
Follicle-Stimulating Hormone (FSH), one of the gonadotropic hormones, is imperative for women’s pubertal development and ovarian/testicular tissues. It stimulates the growth of ovarian follicles and increases the production of estradiol [10]. Besides, estradiol is a steroid hormone and acts as a primary estrogen during the reproductive years. It also controls the processes of female sex characteristics and is responsible for supplying the proper environment for oocytes in the ovary [11]. On the other hand, LDH is an enzyme and a biochemical marker of cytotoxicity, which is involved in the glycolysis process under anaerobic conditions, when the oxygen resources are decreased. It is also organizing some cellular pathways such as cellular proliferation, glucose metabolism, differentiation, angiogenesis, and apoptosis [12].
Studies reported that several natural agents, chemicals or substances such as phosphodiesterase inhibitors, catalase, superoxide dismutase, vitamin E and C, beta-carotene, selenium, N-acetylcysteine, allopurinol, flavonoids, and plant extracts had been used in experimental studies to prevent the I/R injury [3,4,13].
Ozone is a colorless gas consisting of triatomic oxygen (O3) with a cyclic structure [14]. It has been proven that medical ozone has therapeutic effects with its antioxidant, apoptotic, antibacterial, antiviral, antifungal, and anti-inflammatory properties. The medical uses of ozone therapy are lung diseases, neurological diseases, skin diseases, peripheral and cardiovascular diseases, orthopedic diseases, gastrointestinal and genitourinary system diseases [15,16]. Ozone shows its efficiency by activating the antiinflammatory and antioxidant pathways of the body [13].
Most of the related studies in the available literature presented the immunohistochemical and histopathological findings of ovarian I/R injury. However, the hormonal functions and biochemical views of the ovarian torsion-detorsion model were not sufficiently elucidated. We hypothesized that ozone may be used as an alternative treatment option for ovarian injury. This study aimed to determine the effects of medical ozone on the ovarian functions and ovarian morphology in an experimental rat ovarian torsion-detorsion model.
The study was approved by the Institutional Animal Use and Care Committee of Kafkas University (KAU-HADYEK) (Approval date: 10.27.2016 and number: KAU-HADYEK/ 2016-113) and performed according to the “Guide for the Care and Use of Laboratory Animals” principles [17]. The study reporting was done per the CONSORT principles [18].
All rats were housed in an animal room with standard animal cages and were controlled under appropriate environmental conditions (an air-conditioned room at room temperatures and a 12 h light/dark cycle). Twenty female Wistar albino rats, 4-6- weeks old, weighing 200-240 g, and in their estrous phases were used in this study.
Estrus cycles were synchronized with an agonist of Gonadotropin-Releasing Hormone (GnRH) receptor. The peptide was dissolved in 0.1 N acetic acid and then diluted in phosphate-buffered saline so that an injection of 200 μL contained 2 μg of the hormone. This dose was injected subcutaneously at 9:00 AM and 2:00 PM. On the second day after injections, all rats were in diestrus I and continued to be synchronized for at least this first cycle.
The rats were randomly divided into two groups (totally n=20) as follows:
Group 1 (n=10): Torsion and detorsion were performed, followed by ozone-administration (95% oxygen+5% ozone). A total of 8 doses of ozone was administered; four doses for consecutive four days, and four doses for consecutive four weeks.
Group 2 (n=10): Torsion and detorsion were performed without any other intervention. All surgical procedures were performed under 50 mg/kg ketamine anesthesia (intramuscularly). Under aseptic conditions, an abdominal midline incision of 2.5 cm (laparotomy) was applied. All rats were subjected to right unilateral adnexal torsion. The right adnexa, including tubal and ovarian vessels, were rotated by 360-degrees. The rotated adnexa was fixed to the abdominal muscles with a 4/0 silk suture, and the abdominal incision was closed. Six-hours later, under the same anesthesia protocol, laparotomy was performed again to all rats. The same surgical procedure was performed for detorsion; the ovary was counter-rotated to its normal position (Figure 1). After detorsion, rats in Group 1 received 95% oxygen plus 5% ozone gas mixture intraperitoneally at a dose of 0.5 mg/kg. A total of 8 doses of ozone were administered; four doses for consecutive four days, and four doses for consecutive four weeks. At the end of the experiment (a month later), the rats were sacrificed according to the ethical rules with cervical vertebra dislocation, rat blood samples were collected via the tail vein, and ovarian tissue samples were collected for biochemical and histopathological examinations.
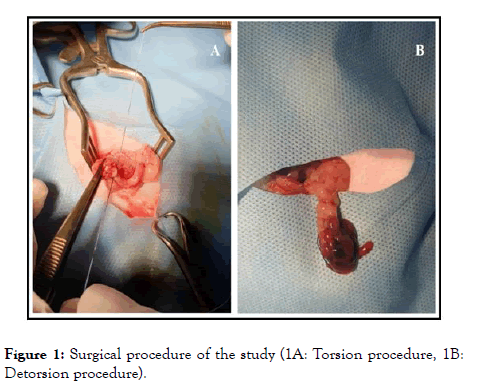
Figure 1. Surgical procedure of the study (1A: Torsion procedure, 1B: Detorsion procedure).
The harvested ovarian tissues were fixed in 10% formalin solution for 72h, dehydrated, cleared in xylene solution and embedded in paraffin wax. The paraffin blocks were sectioned at a five-micrometer thickness using a microtome and stained with hematoxylin and eosin (H and E). Stained histopathologic sections were analyzed under a light microscope (BX46 Clinical microscope, Olympus, Japan) regarding hemorrhage, edema, vascular congestion, follicular cell degeneration, and inflammatory cells. The findings were then scored semiquantitatively for each criterion separately using a scale of 0 to 3 (0: normal; 1: mild; 2: moderate; and 3: severe).
Blood samples of all experimental groups were centrifuged at 1000 rpm at 2-8°C for 15 minutes, and the collected sera were transferred to sterile tubes. The samples were kept at -80°C in a deep freeze until the day of analysis. The concentration of LDH (IU/L), FSH (ng/mL), and E2 (pg/mL) were measured by the Enzyme-Linked Immunosorbent Assay (ELISA) method, using commercially available Rat LDH ELISA kit, Rat FSH ELISA kit (Elabscience, Catalog No: E-EL-R0391), and Rat E2 ELISA kit (Elabscience, Catalog No: E-EL-0065). All samples were run twice in the ELISA assay
For the measurement of the Total Antioxidant Capacity (TAC), the serum samples were first centrifuged at 3000 rpm for 15 min. Then, serum TAC levels (mmol Trolox Eq/l) were determined using spectrophotometric kits. TAC measurements were performed according to a new automated measurement technique developed by Erel [19]. This method measures the antioxidant efficiency of the sample against the potent free radical reactions initiated by hydroxyl radicals.
For histopathological evaluation, semi-quantitative analysis was performed. Five separate microscopic areas were randomly chosen for each section. The mean values of the hemorrhage, edema, vascular congestion, follicular cell degeneration, and inflammatory cells were scored semi-quantitatively. Scaling was done as follows:
A: absent (0%-25% of the tissue), B: minimal (26%-50% of the tissue), C: moderate (51%-75% of the tissue), and D: severe (76-100% of the tissue). The mean values of the histopathologic parameters were calculated as ((A × 1)+(B × 2)+(C × 3) +(D × 4))/(A+B+C+D) and reported as; none (0.00), mild (0.01–1.00), medium (1.01–2.00), severe (2.01–3.00), and extremely severe (3.01–4.00). The scores were interpreted as no injury (Grade 0), minimal injury (Grade 1), moderate injury (Grade 2), and severe injury (Grade 3) [20].
The Statistical Package for the Social Sciences (SPSS, version 22, IBM, Armonk, New York 10504, NY, USA) was used for the statistical analysis. A comparison of variables with normal distribution was made with Student t-test, whereas variables without normal distribution were analyzed using the Mann Whitney U test. The confidence interval was 95% and p-value less than 0.05 was considered as statistically significant.
The histopathological results are reported in Table 1 and Figure 2. Table 1 shows the semi-quantitative assessments of histopathological findings in the ovary tissues of all experimental groups.
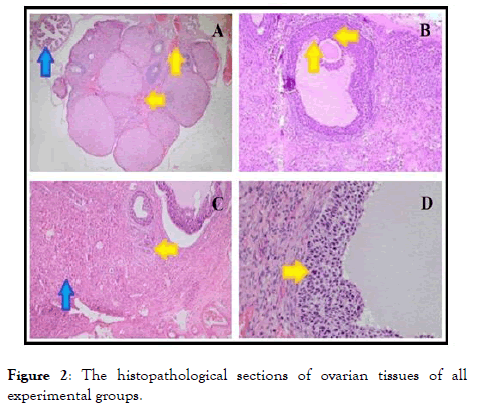
Figure 2. The histopathological sections of ovarian tissues of all experimental groups.
| Groups | Hemorrhage | Edema | Vascular Congestion | Cellular damage | Inflammatory cells | ∑Score |
|---|---|---|---|---|---|---|
| Group 1 (n=10) | 1 | 1 | 1 | 1 | 0 | 4 |
| Group 2 (n=10) | 2 | 1 | 3 | 2 | 0 | 8 |
Table 1: Semi-quantitative assessments of histopathological findings in the rat.
The numbers represent the severity of injury in the related experimental groups regarding hemorrhage, edema, vascular congestion, cellular damage, and inflammatory cells. As seen in Table 1, ozone application leads to a decrease in some histopathological parameters such as hemorrhage, vascular congestion, and cellular damage (apoptosis and/or necrosis). On the other hand, no histopathological changes were detected concerning edema and inflammatory cells.
Aditionally, Figure 2 shows the histopathological changes between ozone-administered and non-ozone administered torsion/detorsion groups. A degenerated follicle structure degenerated corpus luteum, and necrotic cells in the follicle were detected in the ovarian tissue of Group 1 (Figure 2A and 2B).
On the other hand, mild congestion and apoptotic cells in the follicle were detected in ovarian tissues of Group 2, whereas the tuba uterine was morphologically normal in the same experimental group (Figure 2C and 2D).
When the biochemical findings were evaluated, it was seen that Group 1 had the highest level of TAC, while Group 2 had the highest level of FSH (Table 2). A statistically significant difference between the two groups was detected in terms of TAC (p=0.001) and FSH (p=0.002). The E2 and LDH values of Group 1 and Group 2 were close to each other. There were no significant differences between Group 1 and Group 2 regarding E2 and LDH levels (p=0.757, p=0.453, respectively).
| Groups | TAC (mmol/L) Mean ± SD | FSH (ng/mL) Mean ± SD | E2 (ng/mL) Mean ± SD | LDH (IU/L) Mean ± SD |
|---|---|---|---|---|
| Group 1 (n=10) | 4.07 ± 0.28 | 49.68 ± 19.12 | 110.57 ± 8.19 | 882.57 ± 149.46 |
| Group 2 (n=10) | 2.73 ± 0.45 | 123.29 ± 51.18 | 111.97 ± 10.50 | 853.11 ± 66.27 |
| p-value | 0,001 | 0.002 | 0.757 | 0.453 |
Table 2: Comparison of biochemical parameter levels of all experimental groups.
According to the literature, there are only a few experimental studies that investigate the effects of I/R injury on the hormonal functions and morphological views of the ovary [1-3]. In the present study, ovarian functions after the torsion process, and medical ozone administration were evaluated through hormonal, biochemical, and histopathological assays. We revealed that medical ozone positively affects the histopathological markers of cellular damage and increases the antioxidant capacity in the torsion-detorsion model.
The mechanism of ovarian injury after ischemia/reperfusion process is still not precisely understood. However, it has been proved that oxidative stress is one of the most known pathophysiological mechanisms. Oxidative stress-induced by I/R damage is a process that results from an imbalance in the generation of ROS, which may affect some physiological processes (oocyte maturation, fertilization, embryo development, and pregnancy) in the female reproductive life [8,21]. Inflammation, another pathophysiological process, is also induced by ischemia/reperfusion, as well as excessive oxidative stress [5-32].
In conclusion, the I/R injury leads to severe oxidative stress accompanied by inflammatory reactions in all tissues.
Medical ozone administration (ozone therapy) is used as a therapeutic modality for treating and/or eliminating symptoms of several diseases, including ischemic and infectious diseases. Many positive and therapeutic effects of ozone on ischemic tissues ensue via increasing microcirculation and decreasing neutrophil infiltration, platelet aggregation, erythrocyte flexibility, and blood viscosity. As a result, ozone causes an increase in the oxygen level, leading to elevated blood flow in the ischemic tissues with reperfusion. Our findings are by the current literature, that ozone application creates a positive effect on biochemical and histological markers on ovarian torsiondetorsion model in rats.
In parallel, studies reported that different natural agents used for ovarian torsion-detorsion model increased the TAC values.
In our study, the levels of FSH and E2 were compared to evaluate the changes in hormone levels. In group1, serum levels of FSH were significantly reduced. However, no statistically significant differences were detected between the groups in terms of estradiol levels. The postoperative serum levels of E2 in the torsion group were significantly lower than the pre-operative levels. On the other hand, the LDH enzyme levels were decreased in the ozone-administrated group in our study; did not reach statistical significance as well. An increase in the levels of LDH was seen in the ischemic process. The reduction of oxygen leads to anaerobic glycolysis during the ischemic period, then cellular acidosis occurs, and cell death is activated. At that point, increased LDH levels can be detected.
Histopathologically, ozone application leads to a decrease in histopathological parameters which cause hemorrhage, vascular congestion, and cellular damage (apoptosis and/or necrosis). However, in the present study, no histopathological changes were detected regarding edema and inflammatory cells. All ovarian torsion-detorsion model studies generally reported similar histopathological findings. One study showed that Neutrophil infiltration in the torsion groups. In light of these findings, the histopathological results supported that medical ozone administration can prevent tissue damage concerning hemorrhage, edema, vascular congestion, follicular cell degeneration, and inflammatory cell infiltration, whereas, biochemical results were not supportive of this hypothesis. Some parameters were different; however, these differences were not statistically significant. On the other hand, prolongation of the I/R duration may cause a loss of fertility, and this duration shows the significance between studies. Generally, studies reported that 3-hours ischemia is enough to cause biochemical and morphological changes for I/R damage in the ovarian tissue. Since we performed 6-hours ischemia, we think that this duration has severely affected our biochemical results.
In surgical detorsion seems not sufficient to protect the ovarian reserve, and thus, antioxidant/anti-inflammatory treatment options should be evaluated in addition to conservative therapy. In our study, medical ozone, a powerful antioxidant, affected the histopathological parameters and FSH levels in the torsiondetorsion model. We consider that ozone can be used as an alternative treatment option during ovarian detorsion after its effectiveness is confirmed with more comprehensive and molecular studies.
Overall, this study has some limitations. First, the experimental groups of our study could be extended for better comparisons. Control, Sham, and different doses of ozone administration groups could be added, which might have different effects. Second, the methodology of our study could be extended regarding both biochemical and histopathological methods. To detect the apoptosis and/or necrosis, TUNEL and/or NFKB staining techniques could be performed. Additionally, biochemical (malondialdehyde, superoxide dismutase, catalase, lactoperoxidase, and xanthine oxidase) and immunological (TNF-alpha, IL-1-beta and IL-6) markers could be measured to compare the efficiency of ozone on oxidative stress, the antioxidant system, and the inflammatory process.
The authors have no conflicts of interest in this study. The authors alone are responsible for the content and writing of this article.
The authors declare no competing interests.
Citation: Baykus Y, Deniz R, Adali Y, Kara F, Ozturk O, Aydin S, et al. (2019) Therapeutic Effects of Medical Ozone in the Functions and Histopathological Features of the Ovary in an Experimental Torsion-Detorsion Model. Gynecol Obstet (Sunnyvale) 9:510. doi: 10.35248/2161-10932.19.9.510
Received: 27-Sep-2019 Accepted: 30-Oct-2019 Published: 07-Oct-2019
Copyright: © 2019, Baykus Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.