Journal of Pharmaceutical Care & Health Systems
Open Access
ISSN: 2376-0419
ISSN: 2376-0419
Research Article - (2017) Volume 4, Issue 2
Introduction: The study was conducted to evaluate therapeutic efficacy of Coartem® for the treatment of uncomplicated falciparum malaria in Wondogenet Woreda, Sidama Zone, Ethiopia. Since the spread of Plasmodium falciparum, parasite resistance to almost all antimalarial monotherapies is a serious impediment to malaria control. Artemether-lumefantrine (Coartem®) therapy has been in use as the first-line treatment for uncomplicated falciparum malaria since 2004 in Ethiopia. Methods: The study was designed according to WHO study protocol. The study outcomes were classified into Early Treatment Failure (ETF), Late Clinical Failure (LCF), Late Parasitological Failure (LPF) and Adequate Clinical and Parasitological Response (ACPR). Results: Primary study was conducted on ninety-nine P. falciparum mono-infected consenting patients who were enrolled in the 28-day in vivo Coartem® treatment followup study. Based on this, the overall cure rate for Coartem® was 98.9% (PCR uncorrected). The study also demonstrated 4.3% Plasmodium vivax and 2.2% P. falciparum/P. vivax co-infections at the end of followup period. Following Coartem® treatment, fever was cleared rapidly on days 1 and 2 and parasite clearance was high on days 1 and 3. Therefore, the study showed a high therapeutic efficacy of Coartem® for the treatment of uncomplicated falciparum malaria in Wondogenet Woreda. Conclusion: Coartem® had high efficacy for the treatment of uncomplicated falciparum malaria. It also had high efficacy with respect to clearance of fever and elimination of gametocytes within short period of time. The tolerability of Coartem® was very good with persistence of only minor adverse effects. The 1.1% LPF detected by the study and the occurrence of P. vivax/P. falciparum co-infection at the end of 28 follow up days require PCR confirmation.
Keywords: Coartem®; Wondogenet woreda; Cure rate; Malaria; Parasitemia
Malaria is one of the most important infectious diseases in the world and it has been recognized as the most widespread infection in tropical and subtropical areas [1]. Despite its control, the impact of malaria on human population has been continuing to increase [2-4]. An estimated 3.3 billion world’s population are at risk for malaria [5,6]. It is estimated that the number of cases of malaria in 2011 was 216 million. Furthermore, there were an estimated 655,000 deaths each year in the world, of which 91% were in Africa [4,5].
Malaria is also one of the leading causes of morbidity and mortality in Ethiopia. About 68% of the total population is at risk of acquiring malaria infection. The nature of malaria transmission in Ethiopia is seasonal and unstable [7-10].
In Ethiopia, Chloroquine has been the main stay of malaria treatment for more than five decades [11]. In 1999, the FMOH changed the drug policy from CQ to SP, as first line treatment for uncomplicated Plasmodium falciparum malaria. Then, in 2003 a national survey conducted by the FMOH demonstrated a mean treatment failure of 36% for SP [7]. At the same time, initial trial with Artemether/lumefantrine (AL) (Coartem®) provided excellent efficacy with 0% resistance. Based on these data, nowadays Coartem® serves as first line treatment for uncomplicated P. falciparum malaria in 84 countries including Ethiopia [4,12,13].
Research conducted in Japan showed resistance of parasite to AL (Coartem®) treatment. Reports of artemisinin resistance from the Thai- Cambodian border also raised global concern for the long-term efficacy of ACT [14,15].
Report from Ethiopia also shows low-level resistance of malaria parasite to AL (Coartem®) treatment [16]. There are also several reports of AL (Coartem®) resistance which are not yet PCR confirmed [17-19]. The present study is, therefore, conducted to evaluate of therapeutic efficacy of artemether/lumefantrine (Coartem®) against uncomplicated falciparum malaria.
Study area
The study was conducted at Wondogenet Health Center, Sidama Zone, SNNP Region, 272 km South East of Addis Ababa and 24 km South of Hawasa. It is located at latitudes 06°55˙10˙˙ N to 07°04˙10˙˙N and longitudes 38°31˙27˙E to 38°36˙29˙ E and with elevation 1686-2609 m above sea level. Its average annual rain fall was 1663 mm.
The total population of the woreda was 115032, of which more than 85% depend on agriculture. Chat and sugar cane are the major agricultural and marketable products. There are 14 Kebeles in Wondogenet Woreda and 9 of them (Wotera and Kechema, Abaye, Aedo, Baja Fabrics, Aroma, Yuwo, Wush Soyama, Chuko and Kela 01) are endemic to malaria (Wondogenet Woreda and Sidama Zone Health office, 2011).
Sample size determination
The classical statistical methods are still recommended for determining sample size. In the present study, sample size was calculated by considering very low proportion of clinical failure of Coartem® in Ethiopia. Thus, with desired precision of 5% and 95% confidence interval [14].
For p=0.05 and d=0.05 a sample size of 73 would be needed. Then the sample size must be adjusted for the follow up losses and withdrawals expected to be 20% in the study period.
n=(1+0.20) 73=88
Where,
p=Anticipated population proportion of clinical failure (p): 5%;
Confidence level: 95%;
Precision (d): 5%;
n=Number of sample.
Initial clinical evaluation
All study participants who meet the basic enrollment criteria during the screening procedure were evaluated in greater depth by clinical staff. Special care were also taken to detect the presence of early signs of febrile diseases other than malaria, this necessitated to exclude the patients from the evaluation. On Day 0, study participants with sign and symptom of malaria were recruited from OPD of Wondogenet Health center for the following selection criteria.
Inclusion criteria
• Both sexes ≥6 months of age;
• Microscopically confirmed P. falciparum mono-infection, with asexual parasitaemia between 500 and 100,000 asexual parasites/μl;
• Body weight ≥5 kg;
• Fever (axillary temperature ≥37.5°C) or history of fever in the previous 24 h;
• Non pregnant and breast-feeding women;
• Patient living within catchment area (5-10 km radius of the health center);
• Informed consents and agreement to return for all scheduled visits.
Exclusion criteria
• P. vivax/P. falciparum co-infection;
• Hemoglobin <5.0 g/dL;
• Artemether-lumefantrine (Coartem®) intake in the 2 weeks prior to study enrollment;
• Unable to take oral medication or repeated vomiting;
• History of allergic reaction to the drug Coartem®;
• Study participants with severe signs of malaria according to WHO criteria, which included severe anemia defined by hemoglobin <5 g/dL, vomiting more than twice in the past 24 h, recent history of convulsions, unconscious state;
• Severe malnutrition.
Treatment
Study participants meeting all enrolment criteria were received treatment only after the study was fully explained to them and have willingly provided their informed consent. All antimalarial treatments were given by the study team members under observation using established treatment regimens. Food such as soft biscuit was given and patients were ordered to drink milk and eat fatty foods in their home. The fixed dose of Coartem® was given twice daily over 3 days based on weight. Enrolled study participants were observed for at least 30 min after treatment to ensure that they didn’t vomit the drug. If vomiting was occurred within 30 min of treatment, the full treatment dose was repeated. Patients with persistent vomiting were excluded from the study and immediately referred to health facility staff for appropriate management. Once the complete enrollment and treatment procedure is finished, the patient was given schedule for routine follow up visit. The study participants were also informed to return immediately to the assessment team for re-evaluation, if symptoms return at any time during the follow up period, even if it was not a regularly scheduled follow up days.
Specimen collection
Finger-prick blood samples were collected from consenting study participants for malaria parasite identification. A drop of blood was collected for parasite identification in each follow up days and on any unexpected visit, if study participants were ill.
Microscopic diagnosis
Blood films were taken at least eight times for each study participants during the study period (day 0, 2, 3, 7, 14, 21 and 28) and on any unexpected visit. Thick and thin blood smears were made on the same slide from each study participants in each follow up day. Thin smear was fixed by methanol and stained with 3% Giemsa for 30 min. Thick blood film was used to determine parasite density, while thin smear was used for species identification.
Parasite intensity
Number of parasites was determined based on the number of asexual parasites observed against 200 leukocytes, assuming 8,000 leukocytes per μl of blood. The number of parasite was calculated using the following formulas.
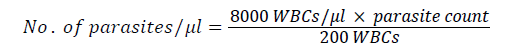
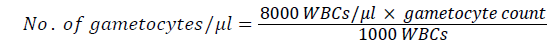
Each blood smear was examined by experienced laboratory technician in Wondogenet Health Center. Smears were declared to be negative only after examining 100 fields in high power objective. The slide was re-examined by senior technician from Ethiopian Health and Nutrition Research Institute. Finally the slides were examined by the investigator for confirmation.
Body temperature measurement
Outcome classifications are dependent on measured body temperature; hence both thermometers and the temperature taking techniques of the clinical staff were evaluated. Any measured temperature below 36°C was repeated.
Hemoglobin determination
Finger-prick blood samples were collected from each patient on day 0 for determination of anemia among the study participants. The fingertip was cleaned with alcohol and prinked with sterile lancet and wiped away the first drop (Table 1). Then, the next drop of blood was used to fill the microcuvette. Finally, the microcuvette was pushed into haemocue analyzer and the displayed value was recorded in g/dl. Anemia is defined as reduction of haemoglobin concentration below normal levels [4].
| Age or gender groups | Non-anemia (g/dl) | Anemia (g/dl) | ||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| Children (0.5-5.0 years) | ≥11.0 | 10-10.9 | 7-9.9 | <7 |
| Children (5.0-12.0 years) | ≥11.5 | 11-11.4 | 8-10.9 | <8 |
| Children (12-15.0 years) | ≥12.0 | 11-11.9 | 8-10.9 | <8 |
| Women, non-pregnant (˃15 years) | ≥12.0 | 11-11.9 | 8-10.9 | <8 |
| Women, pregnant (˃15 years) | ≥11.0 | 10-10.9 | 7-9.9 | <7 |
| Men (˃15 years) | ≥13.0 | 11-12.9 | 8.10.9 | <8 |
Table 1: WHO’s hemoglobin threshold used to define anemia in different age and gender groups.
Follow up evaluation
The WHO 28 days follow up study protocol was used for evaluation (WHO, 2003). The enrolled study participants on day 0 who were successfully treated with the first dose by the study team were given appointment card. It contains patient name, pin number and next scheduled visit date. Patient then asked and advised to come back for treatment in the following 2 days and for 28 follow up days according to scheduled visits on day 3, 7, 14, 21 and 28 and any unscheduled days if patient was ill. At each follow up visit, including day 0 the clinical and laboratory assessment were performed.
Data recording
Patient information was recorded using standardized WHO drug efficacy study record forms [14]. There are four standard record forms:
• Enrollment form: Patient demographic and clinical information;
• Case record forms: Patient clinical and parasitological information;
• Patient record sheet: Laboratory results;
• Follow up cards: Study participants name and follow up dates.
Classification of treatment outcomes
The study outcomes were classified according to WHO standard [14] into the following categories; Early Treatment Failure (ETF) was defined as:
• Development of danger signs or severe malaria on day 1, 2, or 3 in the presence of parasitaemia;
• Parasitaemia on day 2 higher than day 0 count irrespective of axillary temperature;
• Parasitaemia on day 3 with axillary temperature ≥37.5°C;
• Parasitaemia on day 3, ≥25% of count on day 0. Late Clinical Failure (LCF) was defined as:
• Development of danger signs or severe malaria after day 3 in the presence of parasitaemia without previously meeting any of the criteria of ETF;
• Presence of parasitaemia and axillary temperature ≥37.5°C or history of fever on any day from day 4 to day 28 without previously meeting any of the criteria of ETF.
Late Parasitological Failure (LPF) was defined as:
The presence of parasitaemia on any day from day 7 to day 28 and irrespective of axillary temperature without previously meeting any of the criteria of early treatment failure or late clinical failure.
Adequate Clinical and Parasitological Response (ACPR) was defined as:
The absence of parasitaemia on day 28 irrespective of axillary temperature without previously meeting any of the criteria of early treatment failure, late clinical failure, or late parasitological failure.
Adverse effects
All adverse events were monitored and recorded. Treatment emergent symptoms of malaria were defined as adverse events occurring a new or worsening from baseline but occurring before recurrence of parasitaemia.
Data analysis
Data collected from recruited study participants were imported into an Excel spreadsheet and analyzed using Excel data analysis program. SPSS software was used for descriptive statistics and comparing data. Study participants who didn’t meet the follow up protocol were excluded from the analysis.
Characteristics of the study participants
During the study period, a total of 365 patients were examined for malaria infection and 267 were positive for malaria. Of the positive cases, 132 were P. falciparum mono-infection. Of the P. falciparum mono-infected, 7 could not be enrolled in the study for having a parasite load below 500 per microliter of blood. Of the remaining study participants, 4 were excluded from the study due to weakness and unable to attend follow up, 8 were out of the reachable area, 3 had less than 6 months of age, 2 were pregnant and 9 had taken antimalarial drugs before two weeks of the study enrollment.
At last 99 (75%) study participants fulfilled the inclusion criteria were enrolled in the study. Of the 99 cases, 2 were excluded due to repeated vomiting and unable to take full dose of Coartem® treatment. On day 21, 2 study participants were lost follow up and on day 25 and 26 two study participants developed P. vivax/P. falciparum co-infection. Thus, 93 study participants were successfully followed up in the course of the study. On day 21, 93 slides were examined and one positive slide for P. falciparum was found and removed as treatment failure. On the final day (Day 28), slides from the remaining 92 study participants were examined and four P. vivax cases were recorded and taken as a negative slide for data analysis. All study participants that had P. vivax; P. falciparum and P. vivax/P. falciparum co-infection in the follow up days came from the same destination, Wotera and Kechema kebeles.
The baseline characteristics of study participants at submission day are outlined in Table 2. The gender distribution was almost balanced, 49.5 % (n=49) male and 50.5 % (n=50) female. The age stratification showed 13 (13.1%) for under 5 years old; 49 (49.5%) for between 5 and 15 years old and the remaining 37 (37.4%) were adults above 15 years old. Axillary temperature was comparable in the three age groups with mean 38.7°C at enrollment. Hemoglobin concentration was increased with age, ranging from 8.1 g/dl to 17.7 g/dl regardless of parasite load. Parasitic density ranged from 600-96,000 per microliter of blood in all age groups and the highest mean parasite density (23,601.8 per microliter of blood) was observed in children <5 years old followed by 5-15 and >15 years old. Gametocytes at enrollment were observed in 16.2% study participants. The gametocytes that were reported on Day 0 (384 per microliter of blood) and Day 1 (120 per microliter of blood) reduced significantly on Day 7 (16 per microliter of blood), with almost none being recorded on Day 21 and Day 28.
| Characteristics | Parameters | |||
|---|---|---|---|---|
| Age | <5 years | 5-15 years | >15 years | Total |
| Male n (%) | 5 (5.1) | 22 (22.2) | 22 (22.2) | 49 (49.5) |
| Female n (%) | 8 (8.1) | 27 (27.3) | 15 (15.1) | 50 (50.5) |
| Mean age (range) | 2.7 (1.5-4) | 10.3 (5-15) | 29.7 (16-74) | 16.7 (1.5-74) |
| Average temp (°C) (range) | 39.5 (38-40.3) | 38.6 (36.8-40.9) | 38.5(37-41.2) | 38.7 (36.8-41.2) |
| Mean haemoglobin (g/dL) (range) | 10.6 (8.2-13) | 12.2 (8.1-15.5) | 13.7(9.1-17.7) | 12.5 (8.1-17.7) |
| Gametocyte carriagen (%) |
4 (25) | 7 (43.8) | 5 (31.2) | 16 (100) |
| Mean parasite density/μl (range) |
23601.8 (7360-96000) |
11473.3 (600-31600) |
8216.6 (600-46080) |
11425.2 (600-96000) |
Table 2: Baseline characteristics of study participants included in Coartem® in vivo efficacy study against uncomplicated falciparum malaria at the time of enrollment in Wondogenet Woreda, September, 2011 to December, 2011.
Treatment response
In the present study 6.1% study participants were excluded during follow up. Thus, 93 study participants were analyzed at day 28. The cure rate of Coartem® for the treatment of uncomplicated falciparum malaria up to 28 days follow up period was found to be high (98.9%). Adequate clinical and parasitological response was 100% in fewer than 5 years of children and ≥15 years old while it was 98.9% in 5-14 years old. There was no early treatment failure in all age groups but there was one late clinical failure (1.1%) in 5-15 years (Table 3).
| Outcome | <5 years (N=11) | 5-15 years (N=47) | >15 years (N=35) | Total |
|---|---|---|---|---|
| ETF | 0 | 0 | 0 | 0 |
| LCF | 0 | 0 | 0 | 0 |
| LPF | 0 | 1 | 0 | 1 |
| ACPR | 100 | 98.9 | 100 | 98.9 |
| Total | 0.118 | 0.505 | 0.377 | 1 |
Table 3: Cure rate of Coartem® treated uncomplicated falciparum malaria infected study participants on day 28 in Wondogenet Woreda, September, 2011 to December, 2011.
Fever clearance
Seventy nine study participants with fever and the remaining 20 study participants with history of fever participated in the study. Overall, fever clearance was fast following initiation of treatment. Clearance of fever was rapid on day 1 and day 2 and fever had cleared in 74.7% of study participants within 24 h, and 86.9% within 48 h. There was also occurrence of common cold in most study participants from day 21 up to day 28 in the area during follow up.
Parasite clearance
Parasite clearance was rapid. The geometric mean parasite density per μl of blood at day 0 was 11425.2 for asexual parasites and rapidly reduced to 814.5 parasites per μl on day 1. In general, 93.1% and 99.9% of study participants had cleared parasitaemia on day 1 and day 3 respectively, while 100% parasite clearance was observed on day 7.
Gametocyte clearance
Only a very low proportion of study participants (16.2%) had gametocytes on day 0 and the rapidly decreased on subsequent days. From day 14 onwards, the number of study participants with gametocytes was close to zero.
Adverse events following AL (Coartem®) treatment
During the study period clinically detectable drug reactions were reported but no major adverse events were observed throughout the 28 days follow up. Overall, there were several adverse events reported upon taking Coartem®, of which the most common ones were headache in 36.6 % of cases, abdominal pain in 14%, cough in 8.6%, vomiting in 7.5%, joint pain in 5.4%, diarrhea in 3.2%, and dizziness in 2.1% of the cases but no one had developed severe adverse effects (Table 4).
| Symptoms | Day 0 | Day 1 | Day 2 | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|---|---|
| Headache | 86 | 58 | 47 | 34 | 30 | 8 | 3 | 2 |
| Vomiting | 23 | 9 | 7 | 6 | 7 | 1 | 0 | 1 |
| Joint pain | 32 | 16 | 5 | 5 | 4 | 1 | 1 | 1 |
| Abdominal pain | 11 | 11 | 14 | 13 | 13 | 7 | 2 | 4 |
| Diarrhea | 4 | 2 | 6 | 3 | 6 | 3 | 0 | 0 |
| Cough | 12 | 3 | 11 | 8 | 5 | 6 | 2 | 2 |
| Dizziness | 4 | 1 | 5 | 2 | 2 | 3 | 0 | 0 |
| Mouth ulcer | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 |
Table 4: Number of study participants with signs and symptoms of malaria and adverse reactions following treatment of uncomplicated falciparum malaria with Coartem® in Wondogenet Woreda, September, 2011 to December, 2011.
A number of studies conducted worldwide to test the efficacy of Coartem® in Asia and Africa have shown a consistently greater than 95% cure rate with rapid parasite and symptom clearance and significant gametocidal effect [16]. Similarly, the present study determined Coartem® cure rate was very high after 28 days of in vivo follow up study. This finding is consistent with previous efficacy studies in Ethiopia which ranged from 99.1 to 94.7% [16]. On the other hand, it shows disagreement from other reports from Ethiopia with no treatment failure [20,21].
The 98.9% cure rate determined through this study was an improvement over a two years period; from 96.8% cure rate [18] for the same study area. This may be an indication of improvement in drug use practice in the area.
Overall Coartem® efficacy in Wondogenet is not as much different from reports from other parts of Africa (>97% to 96%) [22-24].
Furthermore, results showed excellent 28 day therapeutic efficacy among children (<5 years old) and adults (≥15 years old). This finding was consistent with previous studies [17]. The high cure rate among children could be due to higher milk and fat content in their diet. The time interval between drug administration and food intake, as well as fat content estimate needs further investigation for adult’s ≥15 years old. On the other hand, it is known that high cure rate in adults may be due to the development of acquired immunity that results from repeated exposure to malaria infection which usually acts synergistically with antimalarial drugs [14] to achieve better cure rates than non-immune individuals. Food has a significant effect on the bioavailability of both components of the drug (AL) with an increased absorption when supplemented with fatty ingredients because lumefantrine is a highly lipophilic substance which facilitates drug absorption [22]. Administration of artemether and lumefantrine to healthy volunteers in combination with high fat meal has been shown to increase their bioavailability by two-fold and 16-fold [25]. An increase in treatment success by 15% was observed in Thailand when a fatty diet was co-administered with Coartem® [26].
The possible explanation for detection of P. vivax (4.3%) and P. vivax/P. falciparum co-infection (2.2%) during the follow up days could be initial co-infection with P. falciparum which would suppress P. vivax as hypnozoites or due to new P. falciparum infection. The treatment might also activate the dormant hypnozoites leading to P. vivax relapse or it could be a new infection with P. vivax during the follow up period.
In the present study there was no difference in malaria prevalence between the two sexes among the study participants. This study could be the result of no significant difference in exposure to infection and in health care seeking behaviors of the two sexes in the area. This is comparable with the study conducted elsewhere in Ethiopia [17] and Kenya, Tanzania and Nigeria [27], whereas some studies in Ethiopia have reported higher incidence in males because of frequent travel to malarious areas as laborers [21,28].
The rate of parasite and fever clearance detected in the present study was consistent with reports from previous studies [17,21,24,29,30]. The rapid clearance of fever is known to be associated with the rapid parasite biomass reduction by Coartem®, whereas the highest occurrence of pre-treatment parasitaemia in children <5 years old is associated with the lack of acquired immunity for which adults are known to develop.
Most antimalarial agents other than Coartem® have little or no effect on gametocyte development in P. falciparum infections [23]. The potent effects of Coartem® treatment on gametocyte carriage evident in this study was also reported in previous studies [17,30,31]. The gametocidal action of Coartem® may have been both through clearance of existing gametocytes and killing the asexual stages of the parasite which prevents further gametocyte development.
Most studies [17,21,30] on the safety profile of Coartem® have reported only mild or moderate effects which were also noted in this study.
Coartem® had high efficacy for the treatment of uncomplicated falciparum malaria. It also had high efficacy with respect to clearance of fever and elimination of gametocytes within short period of time. The tolerability of Coartem® was very good with persistence of only minor adverse effects. The 1.1% LPF detected by the study and the occurrence of P. vivax/P. falciparum co-infection at the end of 28 follow up days require PCR confirmation of the genotype of the posttrial parasite population to distinguish drug resistance from reinfection. The impact of Coartem® treatment of uncomplicated falciparum malaria on anemia must be assessed in future studies.
Acknowledgements
We thank Addis Ababa University and Ethiopian Health and Nutrition Research Institute for the financial support. We are thankful to Mr. Moges Kassa and Mr. Ashenafi Assefa for their cooperation during field work and laboratory work and Mr. Fanitu Girma who support laboratory activities. We acknowledge the study participants and administration of the clinic.
Ethical consideration
Ethical clearance was obtained from Addiss Ababa University. Permission was required from Wondogenet Health Offices.
Informed consent
Informed consent was obtained from the study participants. Formal informed consent was obtained from all study participants, in the case of children less than five years age consent was obtained from their parent or legal guardian. The study was fully explained to study participants or parents/guardians, including potential benefits and risks.
Availability of data and materials
The authors decided not to share the data.
Competing interests
The authors declare that they have no competing interests.
Author’s contributions
BP and EB designed drafted and finalized the whole part of the manuscript, additionally the editing process and advisory support was done by BP. DB involved in critically reviewed the manuscript. All authors contributed to the writing of the manuscript and approved the submitted version.