
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2021)
Background: Precancerous Lesions of Gastric Cancer (PLGC) is a common gastrointestinal tract and digestive system disease that lacks effective therapeutic drugs with good curative effects and few adverse reactions. Traditional Chinese medicine (TCM) has the advantages of multiple components, multiple channels, and fewer adverse reactions in the treatment of PLGC. Although Banxia Xiexin Decoction (BXD) demonstrates a good therapeutic effect on PLGC, the pharmacological mechanism underlying its anticancer effect is still unclear.
Methods: We used a network pharmacology strategy, including the construction and analysis of a complex drug-disease network, to explore the complex mechanism of BXD treatment of PLGC. In addition, molecular docking technology was used to preliminarily study the binding ability of the potential active components and core therapeutic targets of BXD.
Results: The network pharmacology results showed 80 targets of BXD that are involved in PLGC. PPI network analysis demonstrated that the top 10 core targets were JUN, TP53, MAPK3, MAPK1, TNF, VEGFA, MAPK14, ESR1, NR3C1, and MAPK8. The GO enrichment analysis results showed that the BXD anti-cancer and anti-inflammatory mechanism mainly involves cellular response to organic cyclic compound, response to toxic substance, response to oxidative stress, cellular response to nitrogen compound, response to inorganic substance, and others. The KEGG analysis results indicated that BXD may regulate 167 pathways such as MAPK signaling pathway and pathway in cancer in the treatment of PLGC. The molecular docking results showed that the binding energies of beta sitosterol with MAPK1, MAPK3, MAPK14, JUN, and VEGFA were less than−7.0 kcal•mol-1, indicating a good docking effect.
Conclusion: This study reflects the characteristics of the mechanism of action by which BXD treats PLGC, which includes multiple components, multiple targets, and multiple pathways, and provides a biological basis for further verification and a novel perspective for drug discover yin PLGC.
Banxia xiexin decoction; Gastric cancer; Molecular docking; Network pharmacology
As a common clinical disease of the digestive system, PLGC is mainly manifested by Gastric and spleen symptoms such as belching and stomach pain. Chronic Atrophic Gastritis (CAG), Intestinal Metaplasia (IM), Dysplasia (Dys) and other gastric mucosa histomathological changes are also present [1,2]. With the development of society, the incidence of Gastric Cancer (GC) is also gradually increasing. Studies show that Gastric Cancer is the fourth most common malignant tumor in the world and according to the latest Research by the International Agency for Research on Cancer (IARC), there was about 1.03 million new cases of gastric Cancer worldwide in 2018, with China accounting for 70% of the total, or about 700,000 people [3]. It is predicted that by 2040, nearly 1.2 million new cases of gastric cancer will occur in China and about 1.1 million deaths will occur, posing a serious threat to global health [4]. The occurrence and development of GC is a complex process of multi-factor crossing, multi-mucosal carcinogenesis and multi-gene variation. Therefore, from normal gastric mucosa-chronic non-atrophic gastritis-chronic atrophic gastritis-gastrointestinal metaplasia-atypical hyperplasia intra- mucosal carcinoma-invasive gastric cancer is to verify the specific process of GC development and evolution based on the hypothesis of Correa [5]. It is suggested that GC is a pathologic model of progressive evolution from precancerous lesion state. The early diagnosis rate is less than 10%, resulting in poor prognosis, extremely low 5-year survival rate, high recurrence rate and easy occurrence of tumor metastasis, which is a serious threat to human physical and mental health. Therefore, secondary prevention of PLGC (preventing or slowing down the progression of the disease through early detection, early diagnosis and early treatment) is the key to preventing and treating GC. Western medicine believes that the occurrence of this disease is often related to dietary factors, Helicobacter pylori (HP) infection and emotional factors. Triple or quadruple therapy is often used for treatment. However, there are problems such as easy recurrence and obvious drug side effects, while the treatment of PLGC with Traditional Chinese medicine, which incorporates the perspectives of physical and mental adjustment and has multicomponent, multi-target, and multi-mechanism characteristics, leads to an individualized diagnosis and treatment program combining differentiation and treatment that has few adverse reactions and can effectively alleviate gastrointestinal tract symptoms associated with PLGC. Among them, BXD has been paid more and more attention by experts and medical staff because of its remarkable effect on PLGC in clinical application.
Banxia Xiexin Decoction, a classic Chinese medicine prescription, one of the National Classic Prescriptions [6] comes from Zhang Zhongjing's Treatise on Febrile Disease in the Eastern Han Dynasty. It is composed of Banxia, Huangqin, Huanglian, Ganjiang, Renshen, Dazao and Gancao. In the original text of Treatise on Febrile Diseases, BXD was used for the misapplication of Xiaochaihu decoction in the method of purging, which caused the damage of middle Jiao Yang, while the evil spirit of Shaoyang disease could not be eliminated and changed into an inward disease. With the deepening of modern research on BXD, its clinical application is often used in the treatment of digestive system diseases such as PLGC and ulcerative colitis. In recent years, both clinical applications and experimental studies have proved that BXD has a definite therapeutic effect on PLGC, and the therapeutic mechanism may inhibit the deterioration of the disease and the cancerization process by regulating the protein expression levels of various signaling pathways and regulating cell proliferation and apoptosis and other phenotypes, but the specific mechanism of action is unclear.
The research of network pharmacology on disease embodies the concept of systematicness, wholeness, and network, which is similar to the concept of wholeness in traditional Chinese Medicine [7,8] Network pharmacology can explain, to a certain extent, the mechanism of action of multicomponent, multi-target, and multi- pathway of TCM compounds in the treatment of diseases [9]. Although BXD demonstrates a good therapeutic effect on PLGC, the pharmacological mechanism underlying its anticancer effect is still unclear. Due to the complex composition of BXD, in this study, the network pharmacology method was used to study this TCM compound. By constructing a multilevel complex network of “Drug-Component-Target-Disease” interactions, the potential material basis and multi-pathway mechanism of BXD in treating PLGC were explored, which provided a theoretical basis for its clinical application. The overall workflow of the study is shown in Figure 1.
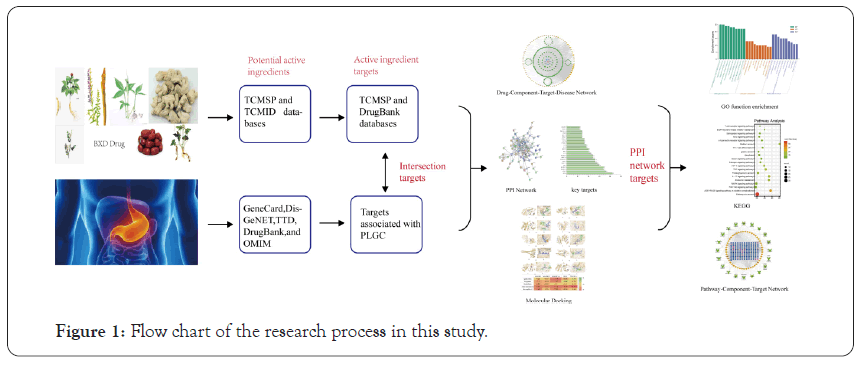
Figure 1: Flow chart of the research process in this study.
Collection of chemical ingredients and targets of BXD
All the components of BXD (Banxia, Huangqin, Huanglian, Ganjiang, Renshen, Dazao and Gancao) were obtained by searching the Traditional Chinese Medicine Systems Pharmacology Database [10] (TCMSP) and Traditional Chinese Medicine Integrated Database [11] (TCMID). The potential active ingredients of BXD were screened by the following characteristics: standard oral bioavailability (OB) ≥ 30% and drug-like property (DL) ≥ 0.18 [12,13]. We searched the targets of the potential active components of BXD in TCMSP and converted the gene names in the UniProt database. The potential chemical targets of Traditional Chinese Medicine were obtained from TCMSP and DrugBank.
Acquisition of targets associated with PLGC
Potential targets associated with “Precancerous Lesions of Gastric Cancer” and “gastritis” was collected from the OMIM databases, Genecards databases, DisGeNET databases, TTD databases and Drugbank databases [14] to search for targets related to PLGC and gastritis to supplement. By intersecting BXD component targets with disease targets, we obtained common targets for both drugs and diseases. Cytoscape software [15] was used to map the drug- component-target-disease network.
Construction of protein-protein interaction network
The shared targets between the drug targets and the disease targets were imported into the Search Tool for the Retrieval of Interacting Genes/Proteins [16,17]. Set the species as "Homo sapiens", the minimum interaction threshold as "Highest Confidence >0.9", the rest as the default Settings, PPI network results were saved in TSV format and imported into Cytoscape 3.6.0 software [18] for visualization and PPI drawing. The "Network Analyzer" plug-in is used to analyze the degree of Network topology properties. The higher the degree, the larger the corresponding node, indicating the more important the target protein is.
Gene ontology and pathway enrichment analysis
Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis for overlapping genes was performed using the Metascape database to identify biological processes. We setted P<0.01,and were performed to save the data results as well as perform visual analysis on the data, and 20 pathways with the largest number of enriched genes were selected. Using Omic Share website map senior bubbles and microscopic letter to build pathways GO enrichment of BP-CC-MF (in Process- cellular component-molecular function).
Molecular docking
The PDB format of the core target protein was downloaded from the RCSB PDB database. We used PyMol 2.4 software to remove water molecules and separate the original ligand from the core target protein. We imported the processed protein targets into Auto Dock Tools 1.5.6 software [19] to hydrogenate, calculate the total charge, and set the atomic type, and saved the files in the “pdbqt” format. The mol2 structures of the corresponding components of the core target were downloaded from the TCMSP database, and Auto Dock Tools was used to set the rotatable bonds and to save the files in the “pdbqt” format. AutoDock-Vina software was used to perform molecular docking. Finally, PyMol software was used to visualize the docking results and establish the docking interaction pattern.
In vivo verification experiment
Establishment of a rat model of PLGC: 6 week-old (170-190 g) SPF Wistar rats, male, 50, purchased from Beijing Vitong Lihua Animal Experimental Technology Co., Ltd., production license of experimental animals: SCXK (Beijing) 2016-0006, qualified. The animal experiment program was approved by the Ethics Review Committee of Experimental Animal Welfare of Shandong University of Traditional Chinese Medicine, Batch No.: SDUTCM2019040203. Animal experiments were carried out in the barrier environment facilities of Experimental Animal Center of Shandong University of Traditional Chinese Medicine. The temperature was 22 ± 2 degrees, the humidity was 50 ± 5%, and the light was alternated at 12:12.The rats were fed with CO60 sterilized diet, and the animals were fed adaptively for 7 days. The control group was fed normally, and the model animals were given 0.02 mol/L MNNG intra gastric administrations combined with abnormal hunger and satiation to replicate the PLGC model for 16 weeks.
Administration and test of experimental indicators: The names and formula proportions of the seven traditional Chinese medicines in BXD are shown in Table S1. All the herbs were purchased from Shandong Medicinal Materials Co., Ltd. At the end of the 16th week of MNNG modeling, the modeling group was randomly divided into BXD high-dose group, low-dose group and model group. BXD was given 24.6 g/kg.d and 11.34 g/kg.d standard intragastric administration, 2 ml/time, twice/day, respectively.
The model group and blank group were given the same amount of normal saline for 8 weeks. After the last administration, anesthesia was performed, blood was collected from the abdominal aorta, centrifuged at 2500 rpm for 15 min, and serum was removed and stored. At the same time, the whole gastric tissue was removed, the stomach was cut along the great curvature of the stomach, a piece of gastric antrum tissue was scraped and fixed in paraformaldehyde to prepare pathological tissue sections, and then HE staining and immune histochemical detection were performed. In addition, two pieces of gastric tissue were cut and placed into enzyme-free tubes and stored in -80°C refrigerator for detection of tissue protein content and gene level.
Acquisition of the active components and targets of BXD
After screening with the set standards of OB ≥ 30% and DL ≥ 0.18, 211 potentially active ingredients were retrieved from TCMSP and TCMID (Table S1), among which 13 were potential active ingredients of Banxia, 36 were potential active ingredients of Huangqin, 14 were potential active ingredients of Huanglian, 5 were potential active ingredients of Ganjiang, 92 were potential active ingredients of Gancao, 29 were potential active ingredients of Dazao, and 22 were potential active ingredients of Renshen. They all have good biological activity. For example, there were stigmasterol in Banxia, Huangqin, Renshen and Dazao (MOL000449, OB: 43.83%, DL: 0.76). As a kind of natural steroid, stigmasterol has strong anti-inflammatory and immunomodulatory effects. It inhibits the release of pro-inflammatory cytokines and protects rats from collagen-induced arthritis [20]. Berberine (MOL001454, OB: 36.87%, DL: 0.78) is one of the main active components of Huanglian and Dazao. It has many functions such as anti-cancer and inhibition of cell proliferation, which can treat a variety of inflammatory and neoplasmic diseases. Berberine can improve the abnormal proliferation of mesangial cells induced by high glucose by regulating ERK/NF-κB signaling pathway [21] and have anticancer effect on osteosarcoma MG-63 cells by inhibiting MAPK pathway [22].
Targets of potential active ingredients in BXD were collected using the TCMSP database and the target genes were annotated using Uniprot data. A total of 159 target genes were obtained, and the "drug-component-target" plot was ploted using Cytoscape 3.6.0, as shown in Figure 2. According to the network diagram, these components all exert therapeutic effects from different genes. For example, baicalein (MOL002714, OB: 33.52, DL: 0.21) is a potential active ingredient of Banxia and Huangqin, which can inhibit the expression of pro-inflammatory cytokines and reduce the occurrence of inflammatory response [23]. β-sitosterol is one of the composition of plant sterols, belongs to tetracyclic triterpene compounds, are the main components of the eukaryotic cell membrane [24], which has antioxidant, cholesterol-lowering, anti- inflammatory, immunomodulatory and anti-tumor effects [25].
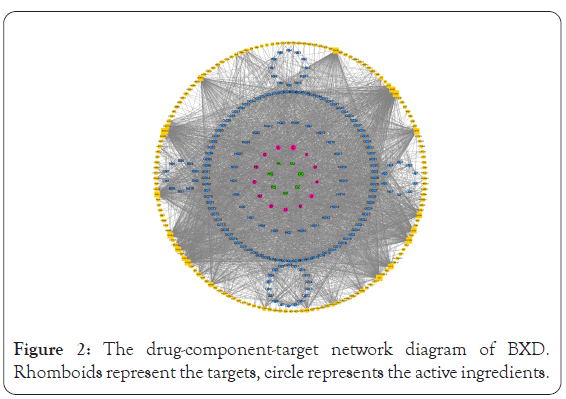
Figure 2: The drug-component-target network diagram of BXD. Rhomboids represent the targets, circle represents the active ingredients.
Construction of the drug-component-target-disease network
After screening with relevance score greater than 1 in GeneCard and score greater than 0.1 in DisGeNET, a total of 2013 PLGC- related targets were retrieved from the GeneCard, OMIM, TTD, DrugBank and DisGeNET databases. After the intersection of drug targets and disease targets, 80 drug disease intersection targets were obtained, namely, the targets of BXD involved in the treatment of PLGC. Cytoscape 3.6.0 software was used to construct the drug- component-target-disease network, and the results are shown in Figure 3. The figure shows that an active ingredient in BXD can correspond to multiple targets, and a target can also correspond to different active ingredients. The same disease may correspond to different targets, and different targets may correspond to different active components of BXD. These results indicated that the antidepressant mechanism of BXD has multicomponent and multi- target characteristics. For example, beta-sitosterol in Huangqin, Ganjiang, Renshen, Dazao, and Banxia share common targets including MAPK14, IL-6, TNF, GSK3β, and PIK3CG to play a potential therapeutic role. The components with a degree value greater than 22 in the network topology analysis were quercetin (degree=60), kaempferol (degree=34), beta-sitosterol (degree=26), wogonin (degree=26), and baicalein (degree=23).
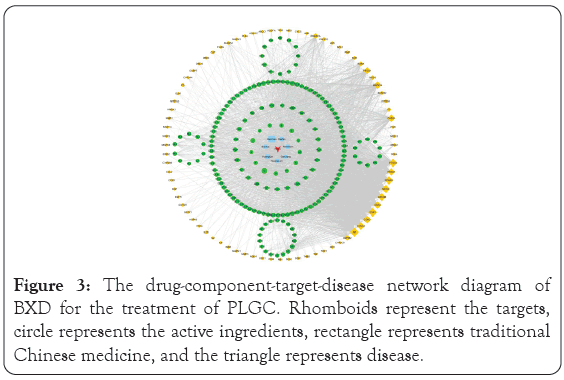
Figure 3: The drug-component-target-disease network diagram of BXD for the treatment of PLGC. Rhomboids represent the targets, circle represents the active ingredients, rectangle represents traditional Chinese medicine, and the triangle represents disease.
PPI network analysis
The overlapping target genes were imported into the STRING database with highest confidence set to 0.9 to obtain a PPI network for the targets. The PPI network results were saved in TSV text format and imported into Cytoscape 3.6.0 software for network topology analysis to obtain the top 10 hub genes. The results of the network topology analysis are shown in Figure 4A. The PPI network contains 80 nodes with 191 edges, in which the circular nodes represent the target proteins, each edge represents the interaction relationship between the target protein and another protein, and the thickness of the edge represents the strength of the interaction force. The top 24 targets obtained on Cytoscape are JUN, TP53, MAPK1, MAPK3, TNF, NR3C1, MAPK14, ESR1, MAPK8, VEGFA, IL6, EGFR, RB1, AR, IL2, RXRA, IL1B, PRKACA, PGR, BCL2, CDK2, PTGS2, NOS3, and GSK3B in Figure 4B. These targets play an important role in the PPI network, suggesting that they are important targets in the treatment of PLGC by BXD. These target genes differ in the number of interactions with other target genes, such as JUN (transcription factor AP-1, 21), TP53 (17), MAPK (17), and TNF (tumor necrosis factor, 15). These associations are meant to be specific and meaningful, that is, the proteins work together to promote shared functions, however, this does not necessarily mean that they are physically bound to each other.
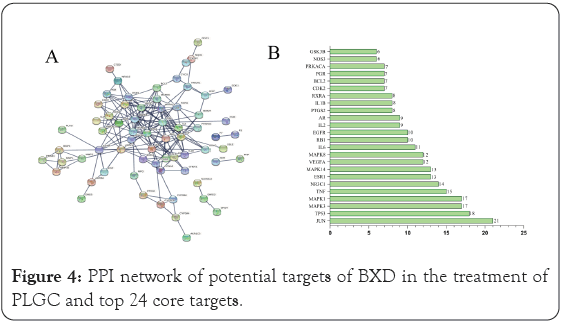
Figure 4: PPI network of potential targets of BXD in the treatment of PLGC and top 24 core targets.
Go enrichment and KEGG analysis of the overlapping targets
GO enrichment analysis mainly involves three aspects-cell composition, molecular function, and biological process. As shown in Figure 5, the biological functions of the targets were analyzed using the Metascape database. The over lapping targets are mainly distributed in cell components such as membrane raft, membrane micro domain, membrane region, vacuole, and vesicle lumen. and are involved in molecular functions such as oxidoreductase activity, protein domain specific binding, cofactor binding, protein kinase activity, phosphortransferase activity, and alcohol group as acceptor. The results indicated that the anti-inflammatory and anti-cancer changes mechanism of BXD mainly involves biological processes such as cellular response to organic cyclic compound, response to toxic substance, response to oxidative stress, cellular response to nitrogen compound, response to inorganic substance, and others. We used the Metascape database to analyze the KEGG pathways of the overlapping targets. A total of 217 pathways were enriched, of which the 167 pathways with statistical significance. The results showed that BXD may achieve antidepressant effects through the Pathways in cancer, AGE-RAGE signaling pathway in diabetic complications, Fluid shear stress and atherosclerosis, HIF-1 signaling pathway, kaposi sarcoma-associated herpesvirus infection, Proteoglycans in cancer, PI3K-Akt signaling pathway, TNF signaling pathway, MAPK signaling pathway, and so on. The top 20 pathway terms with the highest enrichment coefficients are shown in Figure 6. These pathways are closely related to gastric cancer and precancerous changes. Through the analysis of these pathways, BXD plays a therapeutic role in precancerous cancer mainly through anti-inflammatory immunity, inhibition of tumor cell proliferation, promotion of apoptosis, regulation of related signaling pathways and other aspects.
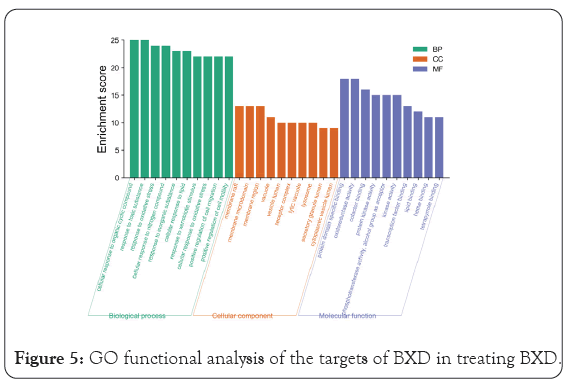
Figure 5: GO functional analysis of the targets of BXD in treating BXD.
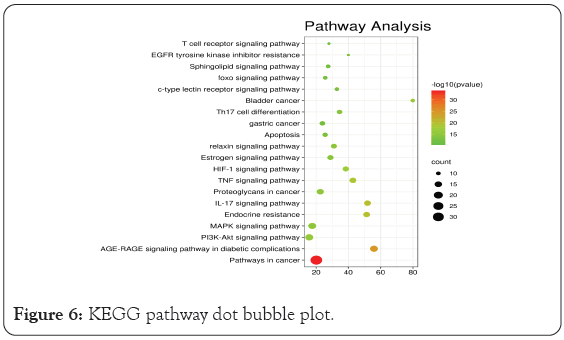
Figure 6: KEGG pathway dot bubble plot.
Analysis of molecular docking results
Quercetin, wogonin, baicalein, kaempferol and beta sitosterol were used for molecular docking with the top 5 hub genes. The molecular docking results are presented in the form of a heat map (Figure 7). The molecular docking software Auto Dock Vina was used to Dock the main compounds, core targets and potential targets of BXD for PLGC. The software evaluates the binding ability of ligand and receptor through binding energy, that is, the value of ΔG obtained after calculation and fitting. A binding energy <-5 is considered to have good binding activity, while a binding energy <-7 is considered to have strong binding activity. It is generally believed that the lower the binding energy of ligand and receptor, the more stable the conformation, and the greater the possibility of action. Lower binding energy indicates a better docking efficiency. The molecular docking results showed that the binding energies of beta-sitosterol with MAPK1, MAPK3, MAPK14, VEGFA, and JUN were less than -7.0 kcal.mol−1, indicating a good docking effect. Quercetin, wogonin, baicalein, and kaempferol have a good docking effect with MAPK14 genes. Wogonin, and kaempferol have a good docking effect with MAPK1 genes.
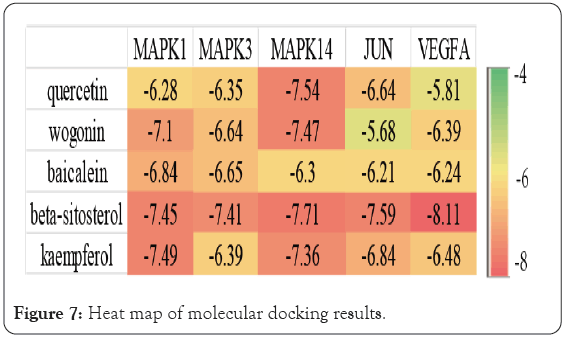
Figure 7: Heat map of molecular docking results.
Diagrams of the interaction structures of the interaction between the active ingredient and the hub genes with good binding energy were drawn. The diagrams are shown in Figure 8 and include 2 different representations designated A, and B. Representation A shows the binding sites of small molecules on the respective proteins. Representation B shows the detailed interactions between the small molecule compounds and the key residues on the respective proteins to show whether the small molecules and proteins interact at specific spatial locations. The active ingredients such as beta sitosterol, kaempferol, quercetin, and wogonin form hydrogen bonds with MAPK14, meanwhile wogonin and kaempferol form hydrogen bonds with MAPK1 respectively. The results showed that beta sitosterol form hydrogen bonds with VEGFA, and beta sitosterol formed other bonds with MAPK1, MAPK3, and JUN genes.
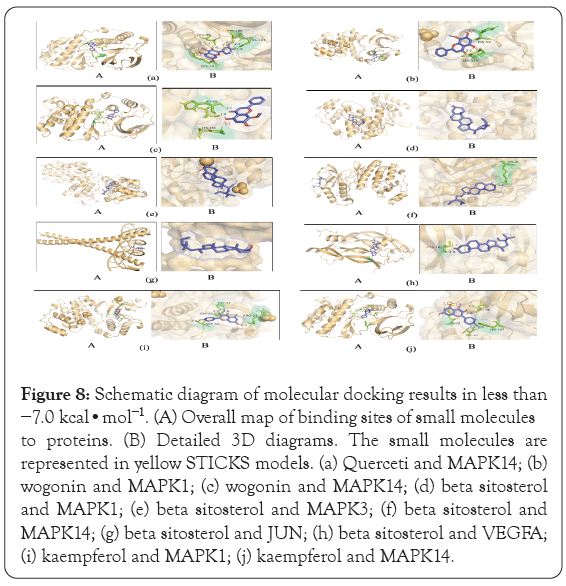
Figure 8: Schematic diagram of molecular docking results in less than −7.0 kcal•mol-1. (A) Overall map of binding sites of small molecules to proteins. (B) Detailed 3D diagrams. The small molecules are represented in yellow STICKS models. (a) Querceti and MAPK14; (b) wogonin and MAPK1; (c) wogonin and MAPK14; (d) beta sitosterol and MAPK1; (e) beta sitosterol and MAPK3; (f) beta sitosterol and MAPK14; (g) beta sitosterol and JUN; (h) beta sitosterol and VEGFA; (i) kaempferol and MAPK1; (j) kaempferol and MAPK14.
PLGC are usually treated according to “epigastric pain”, “PI man” and “noise” in traditional Chinese medicine. Fever, spleen and stomach is deficient, the disorder of qi activity needed for its pathogenesis key, in elected party drugs as relevant to disease pathogenesis and performance is concerned, classic heart square this decoction of traditional Chinese medicine soup with fever should transfer, open bitter drop, fill diarrhea and the formula of characteristic, and in recent years many studies reported found this decoction of heart soup in the treatment of PLGC effect is remarkable, It can comprehensively play the role of drugs from many aspects. In order to explore the therapeutic mechanism of BXD on PLGC, the network pharmacology results showed that, with conditions of OB ≥ 30% and DL ≥ 0.18, 211 main active components of BXD were identified, and these components were predicted to target 80 potential PLGC-related proteins. Five important active components of BXD were identified from drug-component-target-disease network, namely, quercetin, wogonin, baicalein, kaempferol, and beta sitosterol. Quercetin, as a natural anti-tumor drug, can improve the development of gastric cancer and PLGC by up-regulating the expression level of related proteins (such as c-Jun and Cyclin D1 [26], CAV-1 protein [27]) and inhibiting the proliferation of cancer cells by promoting apoptosis [28,29]. Studies have proved that hydroberberine has good analgesic and anti-inflammatory effects, and can replace the use of tetrahydrochloride to a certain extent, but the specific anti-inflammatory mechanism is still unclear [30] Hbaicalein is a flavonoid compound in Scutellaria baicalensis [31]. It has anti- inflammatory, anti-allergic [32], anti-viral [33], anti-convulsion [33], vascular protection [34,35], anti-thrombosis [36] and anti-tumor [37] and other pharmacological effects. The anticancer mechanism of baicalein is mainly realized by inducing cell apoptosis and inhibiting tumor cell proliferation, migration and infiltration [37,38]. Lin et al. [39] found in the cytotoxicity experiment of baicalein that it could not only stimulate the proliferation of natural killer cells, but also increase the killing activity of natural killer cells against gastric cancer cells, effectively inhibiting the growth and reproduction of tumor cells. XH, et al. found effects of baicalein on Wnt/β- catenin pathway protein expression in GC and PLGC. In addition, baicalin can also play a role by inhibiting glucose metabolism of cancer cells, reducing abnormal energy metabolism of gastric cancer cells and inhibiting unlimited proliferation of gastric cancer cells by dose-dependent reduction of glucose intake and lactic acid production in gastric cancer MGC-803 cells as well as prominent target expression during glucose metabolism [40,41]. Baicalein has antioxidant and anti-inflammatory activities, scavenging reactive oxygen species, and reducing the activity of NF-κB to inhibit the release of various inflammatory factors, which has a good effect on diabetes, cardiovascular disease, inflammatory bowel disease, etc. Relevant literature has reported that these active ingredients are significantly associated with PLGC, suggesting that BXD may have an anti-cancert effect through these active ingredients.
PPI network analysis showed that VEGFA, JUN, MAPK1, MAPK3, and other targets have important implications in the treatment of PLGC with BXD. GO function analysis shows that BXD is involved in biological processes that can achieve anti-inflammatory effects, such as cellular response to organic cyclic compound, response to toxic substance, response to oxidative stress, cellular response to nitrogen compound, response to inorganic substance, and others. KEGG pathway analysis showed that BXD mainly regulates 167 pathways such as MAPK signaling pathway to treat PLGC. The MAPK is the main pathway of extracellular signal stimulation to intracellular transport and then induce cell biological processes. Extracellular signal-regulated kinase1/2 (ERK1/2), p38 MAPK, JNK and ERK5 [42,43] are included. Among them, ERK signaling pathway is the most important pathway that mediates cell proliferation, apoptosis and signal transduction, and plays an important role in the occurrence and development of gastric cancer, including mediating growth factors and stimulating activation information through stepwise enzyme reaction. MAPK pathway expression is divided into three levels of enzymic reaction, namely Raf → MEK1/2 → ERK1/2 → ELK-1 step-by-step regulation, of which the first level reaction is Raf subtype, also known as MAPKKK, which is the highest level of composing MAPK pathway. Extracellular Growth Factor (EGF), insulin, and G protein bind to RAF protein and change the chemical structure of the protein by phosphorylation, thus activating MAPKK, and then the active signal is transmitted to MAPKK by phosphorylation of MEK1/2 protein. Through tyrosine and threonine phosphorylation, MEK1/2 finally transits the signal to ERK, and ERK enters the nucleus to cause the phosphorylation of MAPK pathway substrate, which affects the transcription of downstream genes and protein expression forms, and responds to cell proliferation, differentiation, cell viability, cell stress and apoptosis. Dysregulation of MAPK/ERK pathway can lead to neurodegenerative diseases, developmental disorders, metabolic diseases and cancers, among which mutations or abnormal activation of key proteins of this pathway have been found in most tissues of cancer patients, and this signal transduction pathway is involved in the regulation of the occurrence of a variety of tumors, lymphatic metastasis and other processes. Studies have shown that MAPK/ERK pathway gathers various extracellular signals and transits them step by step into the nucleus through phosphorylation activation, and activates a variety of transcription factors, which play an important regulatory role in cell growth, proliferation, differentiation, metastasis and even apoptosis.
According to KEGG pathway enrichment analysis and literature review, we hypothesized that BXD might play a therapeutic role in PLGC mainly through the regulation of the MAPK signaling pathway (Figure 9). The MAPK signaling pathway plays an important role in the treatment of PLGC. Some reports suggest that a pharmacological activity in addition to the abnormal can inhibit cancer cell proliferation and promote apoptosis, also is closely related to the state of MAPK signal pathway of protein expression, including BXD of MAPK signal pathways regulation mainly reflects in inhibiting inflammatory cytokines secretion, promote mucosal protective factors inhibiting cell proliferation apoptosis, etc. Yu et al. observed that BXD inhibited EGFR expression, ERK key enzyme phosphorylation and cyclin 1 regulation in gastric mucosa by intervening EGF synthesis in the gastric mucosa in the study of the treatment of Hp infection gastric mucosa injury, and inhibited cell proliferation. In addition to inhibiting cell proliferation, BXD can also promote cell apoptosis [42-44]. Chunxiu [45] found that BXD may play a therapeutic role by regulating the phosphorylation levels of ERK and P38 in the inflammatory signaling pathway of MAPK in macrophages and promoting cell apoptosis. One of the important mechanisms of BXD in the treatment of PLGC may be achieved by inhibiting the activation expression of Ras protein family, regulating the MAPK cascade reaction, promoting the downstream ERK signaling pathway, affecting the phosphorylation level of ERK protein, and inhibiting the activation of the proto- oncogene ELK-1, so as to inhibit cell proliferation and promote apoptosis.
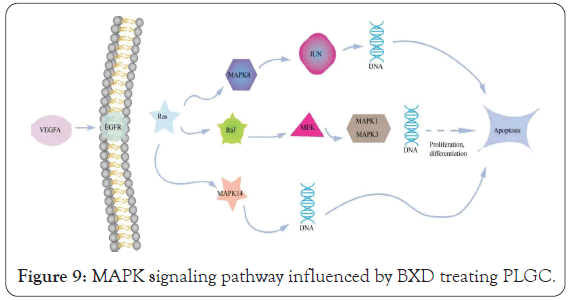
Figure 9: MAPK signaling pathway influenced by BXD treating PLGC.
The network pharmacological and molecular docking results showed that BXD may regulate 167 pathways such as the MAPK signaling pathway and pathway in cancer to treat PLGC. Most network pharmacology studies are based on virtual screening of databases, while TCM compounds can only show a therapeutic role on the basis of in vivo processes such as oral absorption, distribution, metabolism, and excretion. Due to the limitations of network pharmacology, this study needs further experimental verification.
In summary, network pharmacological analysis revealed characteristics of the mechanism of action by which BXD treats PLGC, which involves multiple components, multiple targets, and multiple pathways. BXD is an important modulator of MAPK1, VEGFA, MAPK3, JUN, and MAPK14 through active ingredients such as beta-sitosterol, quercetin, baicalein, and so on. BXD achieves anti-inflammatory and anti-cancer changes effects by regulating 167 pathways, including the MAPK signaling pathway and pathway in cancer. Although some pathways have been shown to be strongly associated with PLGC, some pathways have not yet been shown to be regulated by BXD. Therefore, these pathways provide experimental research directions for the further study of BXD in the treatment of PLGC, especially the MAPK signaling pathway which plays an important role in cell proliferation and apoptosis.
Ethics approval and consent to participate
Not applicable
Not applicable
All data generated or analyzed during this study are included in this published article.
The authors declare that they have no competing interests.
This study was supported by the Key Program of Shandong Province (No. 2016CYJS08A01-6); the first batch of outstanding research and innovation team of Shandong University of Traditional Chinese Medicine. Mechanism and effect evaluation of prevention of major diseases (220316).
Hai-Liang Huang and Tao Han, as corresponding authors with equal contributions, conceived and designed the research. Guo- Xiu Zu performed the research and wrote the paper. Ke-Yun Sun performed the experiments and revised the manuscript. Guo-Xiu Zu, Xiu-Li Zu, Ke-Yun Sun, Ling Li, and Hai-Liang Huang analyzed the data and drew figures. Guo-Xiu Zu and Ke-Yun Sun are equal contributors.
This study was supported by the Key Program of Shandong Province (No. 2016CYJS08A01-6); the first batch of outstanding research and innovation team of Shandong University of Traditional Chinese Medicine. Mechanism and effect evaluation of prevention of major diseases (220316).
Citation: Zu G, Sun K, Li L, Zu X, Han T, Huang H (2021) Therapeutic Targets and Mechanism of Banxia Xiexin Decoction on Precancerous Lesions of Gastric Cancer: Network Pharmacology. J Clin Trials. S12:003.
Received: 12-Aug-2021 Accepted: 26-Aug-2021 Published: 02-Sep-2021 , DOI: 10.35248/2167-0870.21.s12.003
Copyright: © 2021 Zu G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.